Aging is associated with calcium deposits in various cardiovascular structures, but patterns of calcium deposition, if any, are unknown. In search of such patterns, we performed quantitative assessment of mitral annular calcium (MAC) and aortic valve calcium (AVC) in a broad clinical sample. Templates were created from gated computed tomography (CT) scans depicting the aortic valve cusps and mitral annular segments in relation to surrounding structures. These were then applied to CT reconstructions from ungated, clinically indicated CT scans of 318 subjects, aged ≥65 years. Calcium location was assigned using the templates and quantified by the Agatston method. Mean age was 76 ± 7.3 years; 48% were men and 58% were white. Whites had higher prevalence (p = 0.03) and density of AVC than blacks (p = 0.02), and a trend toward increased MAC (p = 0.06). Prevalence of AVC was similar between men and women, but AVC scores were higher in men (p = 0.008); this difference was entirely accounted for by whites. Within the aortic valve, the left cusp was more frequently calcified than the others. MAC was most common in the posterior mitral annulus, especially its middle (P2) segment. For the anterior mitral annulus, the medial (A3) segment calcified most often. In conclusion, AVC is more common in whites than blacks, and more intense in men, but only in whites. Furthermore, calcium deposits in the mitral annulus and aortic valve favor certain locations.
Calcium deposits are commonly found in the aortic valve and mitral annulus. The prevalence of aortic valve calcium (AVC) and mitral annular calcium (MAC) increases with age and in the setting of chronic kidney disease. Associations between MAC and cardiovascular death, stroke, and atrial fibrillation have been previously reported. Furthermore, MAC has been related to the development of mitral stenosis and regurgitation. The amount and distribution of calcium across the aortic cusps and mitral annulus appears to be uneven although this has not been systematically studied. Most studies have looked at AVC or MAC generally, noting its presence or absence. Computed tomography (CT) has the capability, through application of calcium scoring programs, to precisely localize and quantitate calcium in these areas. The aims of this study were (1) to perform a quantitative assessment of the distribution and density of AVC and MAC and (2) to evaluate potential differences in calcium deposition by race and gender.
Methods
We retrospectively analyzed 318 noncontrast axial chest CT scans of subjects aged ≥65. Studies were performed from August 2012 to June 2013 for various clinical reasons, in the inpatient and outpatient settings. Those with mitral or aortic valve replacement were excluded; there were no other exclusion criteria. Demographic and clinical data were gathered from patients’ medical records including age, gender, race, history of coronary artery disease, congestive heart failure, hypertension, chronic kidney disease, and diabetes mellitus. All scans were performed using Brilliance iCT (Philips Healthcare, Cleveland, Ohio) or LightSpeed VCT (General Electric Healthcare, Milwaukee, Wisconsin) scanners. Thin CT slices (0.62 mm to 1.25 mm) were obtained from a “Synapse PACS” system (Fujifilm Medical Systems, USA Inc., Stamford, CT). Specific reconstructions in the planes of the aortic and mitral annuli were created, as outlined in the following, using an “IntelliSpace 3D” workstation (Philips Healthcare) from the axial database of each CT scan.
For analysis of ungated CT scans, we created schematic templates of the mitral annulus and aortic valve on transparent material. This was done using gated cardiac CT scans ( Figures 1 and 2 , respectively). The mitral annulus template ( Figure 1 ) was divided into 6 segments according to Carpentier’s nomenclature. The intercommissural axis was defined as a line through the commissures, with a separate line drawn through the aortic valve and P2. This allowed precise alignment of the template over the mitral annulus. For the aortic valve template, ( Figure 2 ) the left coronary cusp (LCC), right coronary cusp, and noncoronary cusp were depicted in their proper anatomic relation with surrounding cardiac structures.
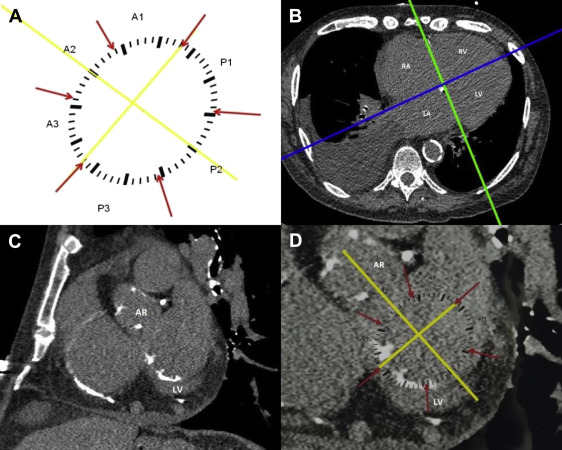

On ungated CT scans, the mitral annulus was defined by creating a cut plane, perpendicular to the 4 chamber plane, through the right and left atrioventricular grooves ( Figure 1 ). The resultant coronal plane displayed the annulus with surrounding landmarks ( Figure 1 ). The mitral annulus was deemed to extend 1 slice (3 to 5 mm) above and 1 slice below the atrioventricular groove. For the aortic valve analysis, 3 orthogonal planes were defined. The oblique plane across the aortic valve annulus was selected using coronal reconstructions ( Figure 2 ). The resultant image depicted the valve in relation to the surrounding landmarks ( Figure 2 ). The area from the attachment of the cusps to the coronary ostia was marked and analyzed. The respective aortic valve and mitral annular templates were then manually applied to the monitor screen, and image magnification was adjusted to allow a proper fit ( Figures 1 and 2 ).
Calcium was defined as focal high-density conglomerates (≥100 Hounsfield units). The location of calcium deposits was assigned using the templates. The Agatston score was calculated in arbitrary units (AUs) for each mitral annular segment and each aortic valve cusp using HeartBeat CS software (Philips Healthcare). Extensions of AVC into the supravalvular and subvalvular regions were manually excluded. All CT scans were analyzed by a single reader to eliminate interobserver variability.
We assessed the prevalence and density of calcium in the aortic valve and mitral annulus across race and gender. We also evaluated the prevalence and density of calcium deposition by aortic valve cusp and mitral annular segment. Data are presented as mean ± SD for continuous variables and as numbers and percentages for categorical variables. We used the chi-square or Fisher’s exact test for normally distributed parameters and the Wilcoxon test for non-normally distributed parameters. A 2-tailed p value of <0.05 was considered to be statistically significant. Statistical analyses were performed using JMP, version 9.0 (SAS Institute, Cary, North Carolina).
This study was approved by the Institutional Review Board of the Albert Einstein Healthcare Network.
Results
The study included 318 subjects aged 65 and older. Baseline characteristics are displayed in Table 1 .
| Variable | Number ( percent ) |
|---|---|
| Age (years ) | 76 ± 7 |
| Men | 154 (48%) |
| Race | |
| Black | 99 (31%) |
| White | 184 (58%) |
| Other | 35 (11%) |
| Hypertension | 211 (66%) |
| Coronary artery disease | 94 (30%) |
| Diabetes mellitus | 83 (26%) |
| Chronic kidney disease | 48 (15%) |
| Congestive heart failure | 52 (16%) |
The prevalence of aortic valve calcium (of any degree) was 50% (159 subjects). The prevalence and density of calcium in the aortic valve cusps was nonuniform ( Figure 3 ); the LCC was more frequently affected than the other 2 cusps although density of deposits did not differ by the cusp. Prevalence of MAC (of any degree) was 43% (137 patients). Distribution of calcium across the mitral annulus was nonuniform. The posterior annulus was more often calcified (36%, 114 patients) than the anterior annulus (17%, 54 patients). In addition, certain segments of the annulus were more often calcified than others ( Figure 4 ). In the posterior annulus, P2 was more frequently calcified than P1 or P3, whereas in the anterior annulus, A3 was more frequently calcified than A1 or A2. When posterior annular calcium was present, average score was 333 ± 583 AU; when anterior annular calcium was present, average score was substantially lower at 90 ± 131 AU. Of the annular segments, P2 tended to be the most heavily calcified ( Figure 4 ).




