Disorders of Cardiac Rhythm and Conduction
Bryan C. Cannon
Christopher S. Snyder
Arrhythmias are common in the general pediatric population and frequently are important clinical problems in patients with structural congenital heart disease. Arrhythmias have a broad spectrum of clinical presentations, ranging from no symptoms to sudden death. There is a wide variety of causes of arrhythmias and an even broader range of treatments. On the most basic level, arrhythmias can be divided into bradyarrhythmias and tachyarrhythmias.
Sinus Node
The sinoatrial node is an extensive, subepicardial structure located on the crista terminalis of the right atrium between the inferior and superior vena cavae (1). The blood is supplied to the sinus node through one of three variants: right coronary artery (60%), left circumflex coronary artery (36%), and both coronary arteries (4%) (2). The main function of the sinus node is electrical; therefore, it is dependent on ion channels. Its cells have unique property of slow, spontaneous phase 4 depolarization known as the pacemaker potential. This phase 4 depolarization can be affected by circulating catecholamines (3). The sinus node also has strong sympathetic and parasympathetic innervation through cardiac ganglionic plexi that directly affect the rate of cellular depolarization. Increased sympathetic tone increases depolarization, while increased parasympathetic tone reduces depolarization. Disorders in any of these areas can result in abnormal sinus function resulting in bradyarrhythmias or tachyarrhythmias.
Anatomy of the Atrioventricular Node
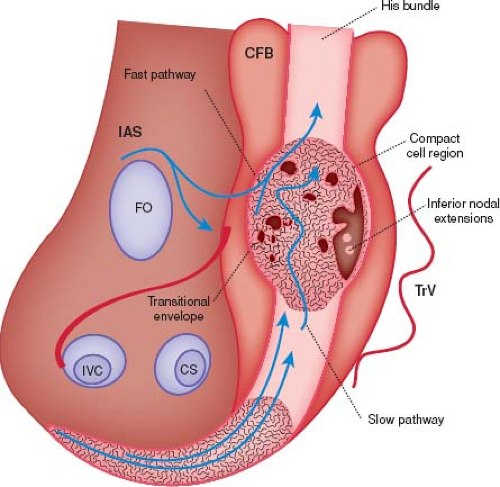
The atrioventricular (AV) node is the electrical connection between the atria and the ventricles. It is a complex structure made up of multiple different components with variable cellular morphology and function. It is generally located at the base of the interatrial septum within the triangle of Koch (bordered by the tricuspid valve annulus, coronary sinus, and tendon of Todaro). As with the sinus node, it has a very rich autonomic innervation with both sympathetic and parasympathetic neurons (4). Its main function is to conduct electrical atrial impulses to the ventricle in a delayed fashion. This delay ensures that the atria have a chance to contract prior to the ventricles being stimulated. When the sinus node fails, the AV node has its own automaticity and therefore can function as an escape pacemaker for cardiac conduction.
The AV node consists of transitional cells, specialized nodal cells (AV node), and the penetrating AV bundle (bundle of His) (Fig. 22.1). The first part of the AV node is the transitional cells. These cells tend to be laid into tracts (the fast and slow pathways) that lead directly from the atrial myocardium into the AV node proper (5). As the cells exit the compact bundle, they begin to organize into larger individual bundles separated by fibrous tissue. As the lower cells progress even more distally, they form the penetrating bundle. The penetrating bundle is engulfed by the central fibrous body (CFB) and also is referred to as the bundle of His. Conduction exits the bundle of His into the ventricles via the left and right bundle branches followed by the Purkinje fibers. The left bundle branch splits into an anterior and posterior fascicle prior to the Purkinje fibers, but the right bundle does not further divide.
Tachycardia
An abnormal mechanism of tachycardia, or tachyarrhythmia, results from an area outside the sinus node, elevating the heart rate or alternatively from abnormal sinus node function. Tachyarrhythmias arise due to abnormal impulse initiation or conduction and may be classified in a multitude of ways. One classification scheme is based upon their mechanism of initiation and propagation. There are three basic mechanisms of tachyarrhythmias: reentry, abnormal automaticity, and triggered activity. It is important to understand these mechanisms because the clinical presentation and features will depend on their underlying mechanism.
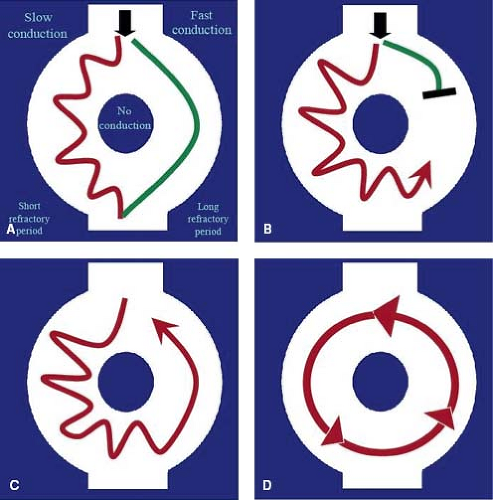
Reentry is the most common mechanism of tachyarrhythmia and is responsible for most supraventricular and many ventricular tachycardias (VTs). For reentry to occur, two distinct conducting pathways must be linked around an area of nonconducting tissue. One limb of the circuit must display slow conduction while the other limb has a long refractory period (Fig. 22.2). This type of arrhythmia can be terminated if one or both limbs of the tachycardia are disrupted. They usually have a rapid onset and offset (usually in a single beat) and may present with rates above 300 bpm. Examples of reentrant tachycardias include AV node reentry tachycardia, accessory pathway–mediated tachycardia, and atrial flutter.
Another cause of tachyarrhythmia is abnormal automaticity. Automaticity is the ability of a cardiac cell to depolarize spontaneously. With abnormal automaticity, there is either abnormally fast activation of cells that exhibit automatic function or the development of spontaneous depolarization in cells that typically do not possess automaticity. Abnormal automaticity often has a metabolic cause (electrolyte disturbances, thyrotoxicosis, hypoxia, ischemia, etc.). It can occur in normal children but is frequently seen in acutely ill children and often exacerbated by intravenous sympathomimetics. The arrhythmias caused by abnormal automaticity show behavior similar to sinus rhythm in that they speed up and slow down according to metabolic and sympathetic changes and are generally refractory to direct current cardioversion. Examples of enhanced automaticity include atrial ectopic tachycardia (AET), junctional ectopic tachycardia (JET), and some forms of VT.
The third, and most rare mechanism of tachyarrhythmia is triggered activity, which has features of both automaticity and reentry. This is the result of an after-depolarization, which is an abrupt change in the membrane potential during the action potential (early after-depolarization) or following full repolarization (delayed after-depolarization). This rapid change in the action potential may serve as a stimulus for a subsequent action potential which can ultimately produce or induce a sustained arrhythmia. These after-depolarizations
are a feature of many drug-induced arrhythmias (such as digitalis toxicity), as well as the initiation of ventricular arrhythmias in long QT syndrome (6).
are a feature of many drug-induced arrhythmias (such as digitalis toxicity), as well as the initiation of ventricular arrhythmias in long QT syndrome (6).
TABLE 22.1 Approximate Normal Resting Heart Rates in the Pediatric Population by Age | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Clinical Presentation
Normal resting heart rates in pediatric patients are age dependent (Table 22.1). One must realize that these are normal resting heart rates and it is frequently challenging in pediatrics to actually obtaining an accurate resting heart rate. Palpation of a child’s pulse or obtaining an ECG can lead to fear and/or anxiety, thereby increasing heart rate through significant sympathetic input. The maximum sinus heart rate that one can achieve is typically 220 bpm minus the patient’s age. Although heart rates above 220 bpm rarely can be sinus in origin, heart rates in this range should warrant evaluation for an abnormal mechanism of tachycardia. Initiation and termination of tachycardia with a single beat also should raise suspicion for an abnormal mechanism of tachycardia. Syncope is a relatively uncommon presentation of tachycardia in the absence of other symptoms suggesting an arrhythmia or underlying channelopathy, although syncope that occurs while actively exercising may be due to an arrhythmic mechanism.
Evaluation
The most important aspect for diagnosis of tachyarrhythmias is the electrocardiogram (ECG or EKG). Documentation of the electrocardiographic rhythm during times of symptoms or tachycardia is the cornerstone for differentiation between “normal” sinus tachycardia and abnormal mechanisms of tachycardia. Ideally, the ECG is performed as a 12-lead rhythm strip but in reality it can be done in any manner capable of recording and documenting the patient’s rhythm. Due to the fact that arrhythmias frequently terminate without warning, immediate documentation should be the second step in managing a patient with a tachyarrhythmia following assessment of the patient’s hemodynamic stability. The most useful part of the ECG in tachyarrhythmia is during times of “wobble;” that is, when the tachycardia starts, stops, or changes. Therefore, a continuous ECG strip should be performed during the time of any intervention to terminate tachycardia, not just before and after.
At times, it can be difficult to diagnose the exact nature of a tachycardia based only on the ECG or rhythm strip alone. In these cases, it may be necessary to use other diagnostic tools to assist with characterization of the tachycardia. Utilizing vagal maneuvers such as the Valsalva or the dive reflex may alter the tachycardia or often times terminate it. The dive reflex is initiated by placing a bag of ice over the entire face (including the nose and mouth) or submersing the patient’s entire face in an ice water mixture for a period of 10 to 15 seconds. Although this is a strong vagal stimulus, it may be alarming to both patient and parent, and care should be taken in small patients to avoid superficial damage to the skin from the ice. Other Vagal maneuvers such as ocular stimulation, carotid massage, gagging, and rectal stimulation are best avoided in the pediatric setting.
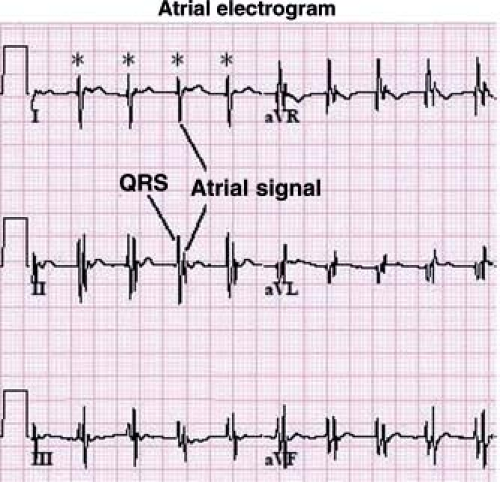
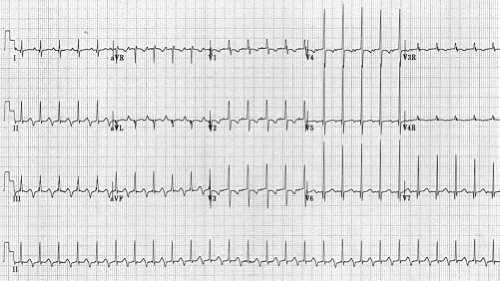
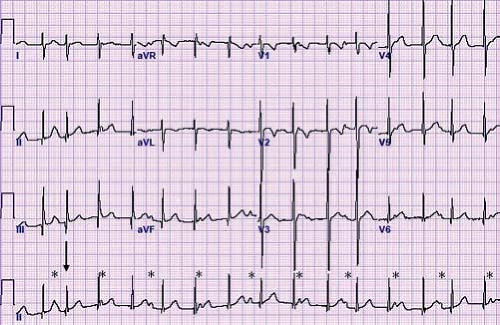
Other methods can also be used to help determine the etiology of tachycardia in cases where the mechanism is not clear. In the postoperative period, atrial temporary pacing wires can be used to obtain a pure atrial signal when it is difficult to determine the relationship of the P wave to the QRS during tachycardia. The two atrial pacing wires are connected to the white (right arm) and black (left arm) leads. This gives a distinct deflection representing atrial activity in lead 1 (Fig. 22.3). Alternatively, one of the wires can be connected to a unipolar lead (V1–V6) to obtain a pure atrial signal in that particular recording on the ECG. In patients without atrial wires, a soft electrophysiology (EP) catheter (either a small transvenous catheter or a specialized catheter made for transesophageal pacing and recording) can be placed in the esophagus and connected to the ECG machine in a similar manner to obtain a recording of the left atrial signal.
Performing a thorough history is also very important in making a diagnosis of a tachyarrhythmia as palpitations are a common complaint in children and teenagers. Differentiation between tachyarrhythmias and sinus tachycardia due to other causes can be challenging. The onset and termination of tachycardias are important historic features. The onset of tachycardia frequently is described as sudden in both sinus tachycardia as well as tachyarrhythmias. The termination of tachycardia typically is gradual in sinus tachycardia. However in a tachyarrhythmia, the termination frequently occurs in a single beat. Dizziness can occur with both tachyarrhythmias and sinus tachycardia. However, if palpitations precede the dizziness, this is more consistent with a true tachyarrhythmia. If dizziness is the first symptom, a tachyarrhythmia is unlikely and the cause is usually sinus tachycardia. Quantification of the heart rate also can be challenging to obtain from a history due to the fact
that it is infrequent that an actual pulse is taken during an episode. Asking the child or parent to tap with his or her hand to approximate heart rate is often a beneficial tool to estimate heart rate. In addition, there are computer apps that can be downloaded to help a patient estimate their heart rate at the time of symptoms. Heart rates that are faster than one is able to count should prompt further evaluation for a tachyarrhythmia.
that it is infrequent that an actual pulse is taken during an episode. Asking the child or parent to tap with his or her hand to approximate heart rate is often a beneficial tool to estimate heart rate. In addition, there are computer apps that can be downloaded to help a patient estimate their heart rate at the time of symptoms. Heart rates that are faster than one is able to count should prompt further evaluation for a tachyarrhythmia.
Many tachyarrhythmias only occur with exercise as the increased catecholamine state may improve conduction in the AV node, making reentrant tachycardias more likely or enhance automaticity in a focal tachycardia. In these particular circumstances, performing an exercise treadmill test may be indicated to elicit an abnormal tachycardia. Several different exercise protocols can be used and the appropriate choice depends on the type of exercise that elicits the tachycardia. The typical continuous escalating protocol (Bruce protocol) may be used or modified to replicate the type of activity that triggers the tachyarrhythmia.
As many patients who have tachyarrhythmias have a normal resting ECG, a 24-hour cardioscan or Holter monitor that records all ECG activities continuously for a 24-hour period is helpful in diagnosing tachyarrhythmias only when symptoms occur at least on a daily basis. Some monitors may be worn for 48 hours or even longer, but it is only beneficial if symptoms occur while the monitor is being worn.
If symptoms happen only occasionally, a number of event monitors may be utilized. The first is a small recording device containing embedded electrodes that has the capability of recording an ECG only when activated. When a patient feels their particular symptoms, the patient places the device on their chest and activates it, which then creates a recording of the rhythm at that time. These recordings can then be transmitted electronically to obtain an ECG tracing during the symptoms. A disadvantage to this system is that the patient must have the loop recorder with them and apply it to their chest during an episode of symptoms to record an event. A second type of event monitor is a looping recorder. This device is connected by ECG monitoring electrodes to the patient and continuously records the rhythm. When the recording switch is activated, it has the capability to store ECG tracings several minutes prior to the activation as well as following the activation. These devices can also be programmed to automatically record the rhythm if the heart rate exceeds or drops below a certain set rate. An external loop recorder may have issues with patient compliance due to the ECG electrodes irritating the skin and the need to have a monitor with leads attached to their chest at all times. Another type of loop recorder may be implanted under the skin to continuously record the cardiac rhythm, thus avoiding the problem of constantly having ECG leads attached. These implantable loop recorders function similar to external loop recorders. They can be set up to record events automatically or can be triggered by the patient with an external activation device. The latest generation of loop recorder can then be automatically downloaded with the information sent through the internet without the patient having to actively participate. This retrieval of events can be done with a pacemaker programmer or via a unit placed in the patient’s home. An implantable loop recorder can be useful in pediatric patients for determining the presence or absence of an arrhythmia during symptoms of syncope, near syncope, and palpitations when conventional diagnostic testing is inconclusive (7). The drawback is the necessity for a minor surgery to implant the device with the need to remove the monitor when it is no longer needed or when the battery life ends (typically around 3 years after implantation). However, newer implantable monitors can be “injected” subcutaneously through an incision around 5 mm wide. Implantable loop recorders are rarely indicated in pediatric patients and should be reserved for cases when it is critical to document the presence and/or characteristics of an arrhythmia. After documentation of the tachycardia, it is then possible to characterize further the arrhythmia and plan a strategy for therapy.
Characterization of Tachycardias
One method for categorizing tachyarrhythmias is to examine the width of the QRS complex and divide the tachyarrhythmias into narrow complex and wide complex. In making this distinction, one must take into account the duration of the sinus QRS, which depends on the patient’s age, underlying congenital heart disease, and history of cardiovascular surgery. In general, neonates have an overall shorter QRS duration than other age groups. In a child <1 year old, a QRS duration of more than 80 ms (two boxes on a standard ECG) should be considered wide. It is always important to view actual printed ECG tracings as a QRS duration of 100 ms may look narrow to the naked eye on an electronic monitor tracing present at the bedside. In general, narrow complex tachycardia originates from an area above the ventricles. The differential diagnosis of wide complex tachycardia, including VT, is discussed later in this chapter.
Narrow Complex Arrhythmias
Sinus Arrhythmia
Although named as an arrhythmia, this phenomenon is related to increases in vagal tone on the heart and is entirely normal. In sinus arrhythmia, the P-wave axis remains normal, but the heart rate increases with inspiration and decreases with expiration. It may be more prominent in younger patients with faster heart rates and is present at some point during 24-hour monitoring in almost all children (8). This variation in rate can be pronounced, but rarely exceeds 100% (e.g., from a rate of 60 to 120 bpm) and if it does, it may signify an abnormality.
Supraventricular Tachycardias
Supraventricular tachycardia (SVT) is the most common sustained arrhythmia in children, with an estimated incidence from 1 in 25,000 to as high as 1 in 250 children (9). The most common age at presentation in childhood is within the first 2 months of life (10).
SVT is defined as an abnormally rapid rhythm that originates proximal to the bifurcation of the bundle of His, is caused by an abnormal mechanism (specifically excluding sinus tachycardia), and does not have flutter waves on the surface ECG. Although the majority patients with SVT have a structurally normal heart, 20% of these patients have some form of structural heart disease (11).
One method for subdividing narrow complex tachycardias is to examine the relationship between the QRS complex and the P wave on the ECG. To do this, a line is drawn halfway between two successive QRS complexes. If the P wave is buried in the QRS complex or is visualized prior to the line between the two consecutive QRS complexes, the tachycardia is considered to be a short RP tachycardia. If the P wave is visualized after this line, it is considered to be a long RP tachycardia. The description of specific types of long RP versus short RP tachycardias is listed in Table 22.2.
Accessory Pathway–Mediated Tachycardia
In the normal heart, the atria and ventricles are isolated electrically from each other by the fibrous annulus of the tricuspid and mitral valves. The only way for electrical impulses to pass from the atria to the ventricles is through the AV node, which penetrates through the central fibrous body to conduct electrical impulses. An accessory pathway is an additional electrical conduction pathway from the atria to the ventricles. This pathway results from a defect in the fibrous annulus as the embryonic atria and ventricles become electrically isolated. This process occurs in all fetuses during cardiac development but regresses in most cases. Persistence of one of these connections creates an accessory pathway. Accessory pathways are
derived from either normal ventricular myocardium or specialized conduction tissue. Accessory pathways can conduct antegrade only (as in Mahaim fibers), both antegrade and retrograde (Wolff–Parkinson–White [WPW]), or retrograde only (unidirectional retrograde accessory pathway [URAP]).
derived from either normal ventricular myocardium or specialized conduction tissue. Accessory pathways can conduct antegrade only (as in Mahaim fibers), both antegrade and retrograde (Wolff–Parkinson–White [WPW]), or retrograde only (unidirectional retrograde accessory pathway [URAP]).
TABLE 22.2 Characteristics of Narrow Complex Tachycardia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
There are two different mechanisms by which accessory pathways can cause reentrant SVT. In an orthodromic tachycardia, the conduction is antegrade through the AV node to the ventricle and then retrograde back up through the accessory pathway to the atrium (Fig. 22.4A). This is the usual form of accessory pathway–mediated tachycardia and results in a rapid rate with a narrow QRS complex. Antidromic tachycardia is the other mechanism of tachycardia (Fig. 22.4B). In this form of tachycardia, conduction is antegrade or down the accessory pathway to the ventricle then
retrograde back up the AV node to the atrium and can only occur in accessory pathways with antegrade conduction. Because the activation of the ventricle is through the accessory pathway and not the AV node, this type of tachycardia is always a wide complex tachycardia. The activation of the ventricle in antidromic tachycardia has a similar pattern as the morphology of preexcitation seen on the surface ECG in sinus rhythm. Of note, in both types of tachycardia all four components (ventricle, accessory pathway, atrium, and AV node) are critical for maintaining the reentrant tachycardia circuit, and the tachycardia may be terminated in any one of these four limbs.
retrograde back up the AV node to the atrium and can only occur in accessory pathways with antegrade conduction. Because the activation of the ventricle is through the accessory pathway and not the AV node, this type of tachycardia is always a wide complex tachycardia. The activation of the ventricle in antidromic tachycardia has a similar pattern as the morphology of preexcitation seen on the surface ECG in sinus rhythm. Of note, in both types of tachycardia all four components (ventricle, accessory pathway, atrium, and AV node) are critical for maintaining the reentrant tachycardia circuit, and the tachycardia may be terminated in any one of these four limbs.
Wolff–Parkinson–White
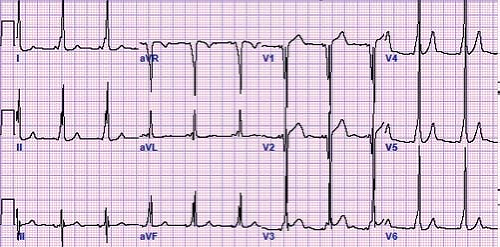
In 1930, Drs. Wolff, Parkinson, and White described an ECG syndrome consisting of a functional bundle branch block and a short PR interval in healthy people with paroxysms of tachycardia (12). This syndrome now bears their names. WPW syndrome or ventricular preexcitation consists of three findings: a short PR interval, a slurred upstroke of the QRS complex (also known as a delta wave), and a QRS complex of longer duration than normal for age during sinus rhythm (Fig. 22.5). These findings may not be found in all leads, although the mid-precordial leads (V2–V4) may be the most sensitive. Other subtle clues to the presence of WPW on the baseline ECG are left axis deviation, the absence of a Q wave in lead V6, abnormally wide Q waves in the limb leads (“pseudoinfarction pattern”), a junctional escape beat with a different QRS morphology, and accentuation or loss of preexcitation with a premature atrial contraction (PAC). The ST or T waves can also be abnormal due to the abnormal depolarization caused by the accessory pathway. The EP definition of preexcitation or WPW is a His to ventricle (HV) interval of <35 ms measured from this His bundle recording to the earliest delta wave signal on the surface ECG.
The incidence of WPW is 0.1% to 0.5% among the general population and is more prevalent in men than women (13,14,15). However, the true incidence of WPW may be much higher because many patients are asymptomatic and therefore undiagnosed. Although asymptomatic patients do not technically meet the definition for WPW “syndrome” (because they do not have tachycardia), these patients are generally labeled as having WPW, although a more correct description would be ventricular preexcitation.
WPW has a known association with congenital heart disease, ranging from 9% to 32% of patients, so an echocardiogram generally is indicated after confirming the diagnosis (10,16,17,18). The structural cardiac diseases known to be associated with WPW include Ebstein anomaly of the tricuspid valve, congenitally corrected transposition of the great arteries (ccTGA), and hypertrophic cardiomyopathy; noncardiac associations include glycogen storage disease and tuberous sclerosis. The majority of WPW cases tend to be sporadic in nature, but in a small minority of these patients (3%), there is an identifiable affected first-degree relative, and familial cases have been reported (7p3 chromosomal association) among others (19). It has been linked to the LAMP2 gene causing Danon disease associated with a dilated cardiomyopathy. Approximately 9% of WPW patients have more than one accessory pathway (20). The incidence of multiple pathways may be as high as 20% in patients who have an underlying congenital heart disease (21).
WPW may contribute to the cause of arrhythmic substrates in two different ways. The first of these is participating in one of the limbs in a reentrant SVT. The second is conduction to the ventricle during atrial fibrillation. Many patients will present with symptomatic SVT, but some will have WPW discovered on an ECG done for other reasons. However, a significant percentage of incidentally discovered patients with WPW will develop an arrhythmia—15% after a mean follow-up of 37.7 months in a large Italian study (22). In higher-risk groups, those with rapidly conducting accessory pathways or multiple pathways, this may be as high as 44% (23). In a large study of military personnel followed over 20 years, 23% with constant preexcitation developed reentrant SVT compared to 8.3% who only exhibited intermittent preexcitation (24).
In patients with WPW, episodes of SVT decrease in frequency over the first year of life in >90% of patients. However, SVT recurs in approximately 30% at an average age of 7 to 8 years (18). Furthermore, there is evidence that in the first year of life the accessory pathway loses anterograde conduction in as many as 40% of patients with SVT becoming noninducible in a similar percentage, suggesting loss of retrograde conduction as well (25). However, if a WPW pattern persists on the patients’ ECG, there is up to a 29-fold increased chance of SVT recurrence, requiring additional antiarrhythmic therapy to control SVT (26). If WPW patients present with SVT after the age of 1 year, >90% of patients will have a recurrence (27).
Patients with WPW rarely present with symptoms of heart failure from the hemodynamic effects of preexcitation alone. This is presumed to be because of dyssynchronous ventricular contraction associated with an extremely preexcited rhythm (28). These patients often have an improvement in their ejection fraction and decrease in their symptoms after ablation of their accessory pathway (29) as well as reverse left ventricular remodeling after elimination of ventricular preexcitation (30).
Patients with WPW are also at risk of developing paroxysmal atrial fibrillation. There are two likely mechanisms by which patients develop atrial fibrillation: one mechanism is reversible and directly
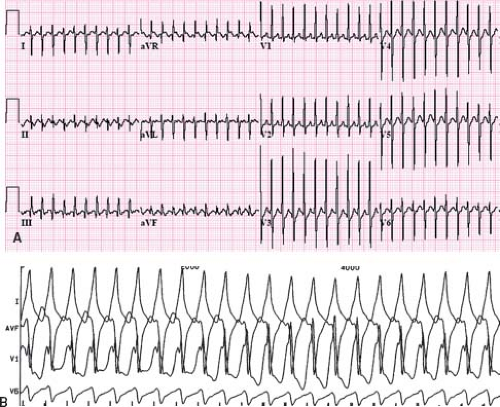
related to the accessory pathway, while the other is intrinsic and accessory pathway–independent atrial vulnerability (31). Just as patients with multiple accessory pathways may be at a higher risk of SVT, they are also at a higher risk of developing atrial fibrillation (32). Atrial fibrillation as a presenting symptom occurs in up to 6% of patients (33). Atrial fibrillation in patients with WPW presents as a characteristic irregularly irregular wide complex tachycardia (Fig. 22.6). This irregularity of the rate tends to separate this arrhythmia from the major other forms of VT, which tend to have a relatively constant cycle length. Rapid conduction down the accessory pathway to the ventricles may precipitate VT or fibrillation with resultant hemodynamic collapse (34). Adenosine, digoxin, and calcium channel blockers are relatively contraindicated in WPW patients in atrial fibrillation because they promote conduction through the accessory pathway rather than the AV node, precipitating ventricular arrhythmias. Direct current cardioversion is the preferred therapy, especially in the presence of rapid conduction down the accessory pathway.
related to the accessory pathway, while the other is intrinsic and accessory pathway–independent atrial vulnerability (31). Just as patients with multiple accessory pathways may be at a higher risk of SVT, they are also at a higher risk of developing atrial fibrillation (32). Atrial fibrillation as a presenting symptom occurs in up to 6% of patients (33). Atrial fibrillation in patients with WPW presents as a characteristic irregularly irregular wide complex tachycardia (Fig. 22.6). This irregularity of the rate tends to separate this arrhythmia from the major other forms of VT, which tend to have a relatively constant cycle length. Rapid conduction down the accessory pathway to the ventricles may precipitate VT or fibrillation with resultant hemodynamic collapse (34). Adenosine, digoxin, and calcium channel blockers are relatively contraindicated in WPW patients in atrial fibrillation because they promote conduction through the accessory pathway rather than the AV node, precipitating ventricular arrhythmias. Direct current cardioversion is the preferred therapy, especially in the presence of rapid conduction down the accessory pathway.
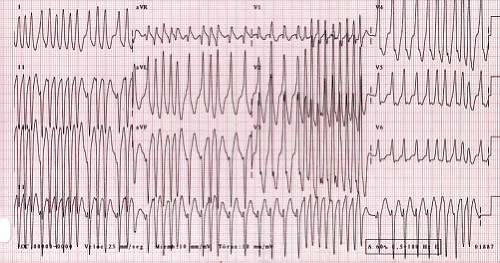 Figure 22.6 Atrial fibrillation in a patient with WPW. This creates an irregularly irregular wide complex tachycardia characteristic of this arrhythmia. |
The incidence of both malignant WPW syndrome resulting in cardiac arrest and WPW causing SVT is higher in patients with syncope than in patients who are otherwise asymptomatic (35). Therefore, syncope in a patient with WPW should prompt rapid referral for further diagnostic testing.
Risk Stratification
Risk stratification in patients with WPW may be beneficial, even in asymptomatic individuals, because sudden cardiac death can be the first presentation of WPW syndrome in up to 1.4% of patients (22). However, several studies indicate that the overall sudden cardiac death rate is low, on the order of 0.0015 per patient-year and that sudden cardiac death is extremely rare in asymptomatic patients at their time of diagnosis (36). Rapid antegrade conduction down the accessory pathway during atrial fibrillation is the primary risk factor for sudden cardiac death. Tachyarrhythmia inducibility and multiple accessory pathways also are risk factors for potentially life-threatening arrhythmic events (34). The risk of sudden cardiac death in patients younger than 8 years old is very small and is minimal in patients younger than 5. As invasive and noninvasive testing in this young age group may present challenges, it is generally accepted to wait until ages 5 to 8 years for formal risk assessment. In a study by Bromberg et al. (37), cardiac arrest was the only distinguishing clinical feature between high- and low-risk groups and the first manifestation in 80% of the children of an accessory pathway that can precipitate a life-threatening arrhythmia. As patient history is not helpful in distinguishing between risk groups in WPW, other methods of risk stratification need to be performed. Even in patients with no symptoms, risk stratification may be indicated. A consensus statement endorsed by the Heart Rhythm Society, Pediatric and Congenital Electrophysiology Society, American Academy of Pediatrics, American College of Cardiology, American Heart Association and Canadian Heart Rhythm society for management of asymptomatic young patients with WPW was published in 2012 (38).
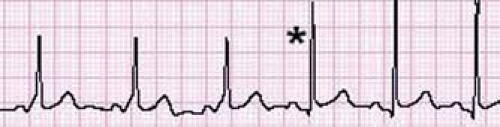
The best method of noninvasively stratifying patients with WPW by risk is to perform an exercise treadmill test. If there is loss of preexcitation, in a single heartbeat, this suggests a low-risk accessory pathway (Fig. 22.7) (39,40). The loss of preexcitation must occur in a single beat, rather than gradually, in order to classify the pathway as low risk based on the treadmill test (41). Although in general intermittent preexcitation seen either on an ECG or 24-hour cardioscan monitor is a predictor of poor anterograde conduction through the accessory pathway, this finding has been observed in some patients who have experienced sudden cardiac arrest (42).
The test that has held up as the best predictor for patients at risk for sudden death is the measurement of the shortest preexcited RR interval (SPERRI) during atrial fibrillation. This measurement can be performed on an ECG of a patient who presents in atrial fibrillation. Atrial fibrillation also can be induced by rapid atrial pacing from a transesophageal pacing probe (43) or by using a single transvenous pacing catheter in the atrium. During atrial fibrillation, two QRS complexes that show preexcitation are located and the shortest interval between two consecutive beats is measured in milliseconds. A SPERRI of <250 ms and especially <220 ms is more commonly seen in patients with WPW who have experienced cardiac arrest (37,44). These patients are candidates for ablation, or medical therapy to alter the conduction properties of the accessory pathway if ablation is not possible. An EP study is actually more useful to identify those at low risk of sudden death as the overall risk of sudden death in WPW is low. As many accessory pathways, particularly septal pathways, may augment their conduction in the presence of catecholamines, it may be beneficial to perform the risk assessment while using an
isoproterenol infusion (45). It is controversial whether the risks of performing an ablation of an accessory pathway (particularly one located in a high-risk location) outweigh the true risk of an arrhythmic sudden death, but eliminating conduction via catheter-based technology should be strongly considered in rapidly conducting pathways.
isoproterenol infusion (45). It is controversial whether the risks of performing an ablation of an accessory pathway (particularly one located in a high-risk location) outweigh the true risk of an arrhythmic sudden death, but eliminating conduction via catheter-based technology should be strongly considered in rapidly conducting pathways.
Concealed or Unidirectional Retrograde Accessory Pathway
A patient may have an accessory pathway even if the surface ECG shows no overt manifestation. This type of accessory pathways conducts only from the ventricle to atrium and is known as a unidirectional retrograde accessory pathway or URAP or concealed accessory pathway. Although the resting ECG is normal, these accessory pathways are often the cause of a reentrant SVT. The therapy and management of these accessory pathways are similar to those for WPW, but because there is no antegrade conduction, these patients are not at risk for sudden death from rapid conduction in atrial fibrillation.
Permanent Junctional Reciprocating Tachycardia
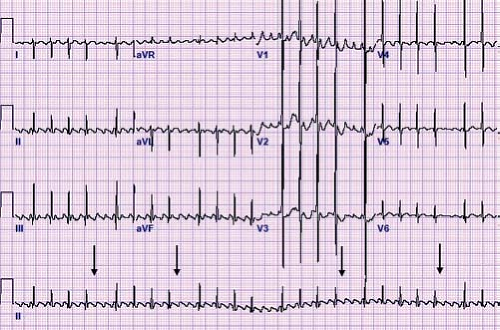
A unique type of accessory pathway–mediated tachycardia is the permanent form of junctional reciprocating tachycardia (PJRT). Initially, this tachycardia was thought to arise from the AV junction and thus was named a junctional reciprocating tachycardia. Later investigation actually determined this to be caused by a slowly conducting, retrograde only accessory pathway, but the name PJRT has persisted. A PJRT fiber is generally located in the posterior septum on the right side of the heart, but PJRT fibers have been described in the midseptum or even on the left side of the heart. Because these fibers conduct slowly, it presents as a long RP tachycardia where the P wave easily is visible on the surface ECG. The ECG in tachycardia has a classic morphology with deeply negative P waves in the inferior leads II, III, and aVF (Fig. 22.8). This type of tachycardia presents at less than 1 year of age about 60% of the time and is incessant in about half of patients (46). However, because of its relatively slow rate (140 to 200 bpm) it may be difficult to appreciate during routine examinations, particularly in newborns and infants. When PJRT is incessant, it can lead to a tachycardia-induced cardiomyopathy if untreated (47).
Mahaim Fibers
Mahaim fibers are a special type of accessory pathways that bypass at least part of the AV node. These fibers connect either the atria to the fascicles (atriofascicular fibers), the bundle of His to the ventricles, the fascicles to the ventricles, or the atria to the bundle of His. These fibers generally are located on the right ventricular side of the heart but have been described on the left ventricular side. These pathways have unique properties similar to those of the AV node in that they often possess decremental conduction (conduct slower at a faster rate) and are typically catecholamine sensitive. Unlike other accessory pathways, these fibers may have automaticity and are capable of depolarizing spontaneously (48). Tachycardia may result from this spontaneous depolarization or from reentrant tachycardia using the Mahaim fiber as the antegrade limb and the AV node as the retrograde limb (i.e., antidromic tachycardia). Due to their right-sided location, the patient’s ECG has a left bundle branch block pattern during tachycardia. The surface ECG in normal sinus rhythm, has as a relatively normal PR interval with a slurred upstroke of the QRS resulting from ventricular depolarization through the Mahaim fiber (49). These fibers often times have only intermittent conduction, and although Mahaim fibers conduct antegrade, their risk for rapid ventricular conduction during atrial fibrillation likely is not present and therefore do not present a risk for sudden cardiac death.
Automatic Focus (Atrial Ectopic Tachycardia)
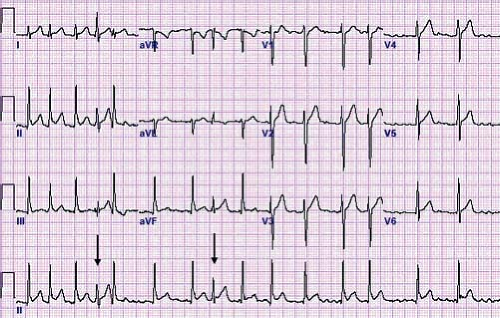
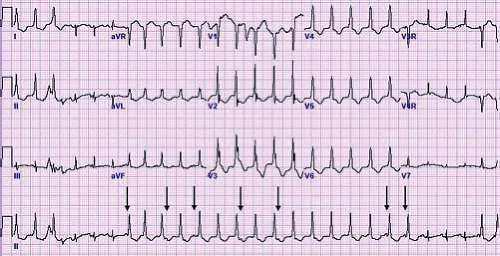
Atrial ectopic tachycardia (also known as ectopic atrial tachycardia) is caused by an abnormal focus of cells in the atria distinct from the sinus node that spontaneously depolarize faster than the underlying sinus node. Automatic focus atrial tachycardias account for 4% to 6% of SVTs and are often incessant resulting in significant cardiac dysfunction (50). The atrial tachycardia rate may increase or decrease depending on sympathetic tone. Distinct P waves typically are visible typically in a long RP pattern. The P waves typically have an abnormal morphology and may be either peaked or notched (Fig. 22.9). First-degree AV block or even Mobitz type I second-degree AV block (Wenckebach) may be present and provide an important clue to the presence of an abnormal tachycardia (51). Common foci for AET include the right atrium along the crista terminalis, the right atrial appendage, and the pulmonary veins (52). The P-wave axis in tachycardia frequently is different than that during normal sinus rhythm, unless it originates near the sinus node or right upper pulmonary vein. When the focus is near the sinus node it shows as
an upright P wave in lead I and aVF similar to normal sinus rhythm, but the P wave frequently is completely negative (rather than biphasic) in lead VI, thus providing a clue to the presence of an ectopic atrial focus. In up to one-third of cases, multiple atrial tachycardia foci are the source of the tachycardia (53).
an upright P wave in lead I and aVF similar to normal sinus rhythm, but the P wave frequently is completely negative (rather than biphasic) in lead VI, thus providing a clue to the presence of an ectopic atrial focus. In up to one-third of cases, multiple atrial tachycardia foci are the source of the tachycardia (53).
The atrial rate in AET frequently exhibits periods of speeding up and slowing down which distinguish this automatic focus tachycardia from the onset and termination in a single beat seen in reentrant tachycardias. This “warming up” and “cooling down” frequently happens rapidly over a period of only a few beats but may be gradual over a period of minutes. Occasionally, AET manifests initially as a wide QRS tachycardia that returns to a narrow complex rhythm after several beats. These initial wide beats occur due to the rate exceeding the refractory periods of one of the bundle branches at the onset of tachycardia resulting in abnormal conduction (aberrancy) resulting in a wide QRS. However, the bundle branches eventually accommodate to the faster rate and the QRS narrows. Patients that present with both a wide and narrow complex tachycardia at the same rate are likely to have an atrial tachycardia that conducts aberrantly rather than two different mechanisms of tachycardia. Adenosine can be used to assist with diagnosing AET as it blocks the AV node usually without affecting the ectopic focus, and the arrhythmia does not terminate. However, some ectopic foci are adenosine sensitive, particularly those originating around the AV node (54). Cardioversion and overdrive pacing may temporarily suppress AET only to have it almost immediately resume at its initial rate or faster due to an increase in catecholamines. Antiarrhythmic medications may be necessary to get the tachycardia under acute control.
Spontaneous resolution of AET is unlikely in children older than 3 years (55). For this reason, cardiac catheterization with ablation techniques can be considered as a first-line of therapy in older pediatric patients although medical therapy can be effective at controlling symptomatic tachycardia episodes. In contrast, children younger than 6 months of age have a high incidence of spontaneous resolution of tachycardia with a low long-term incidence of recurrence. It is therefore advisable to attempt medical control in these patients until the tachycardia resolves if the tachycardia is sustained or very rapid. Slower (<220 bpm), self-limited, asymptomatic atrial tachycardias may not require therapy if the ventricular function is normal, although these patients require close follow-up.
Some foci of AET that are particularly difficult to control with medications alone are caused by hamartomas within the atrial myocardium. If reasonable control of the arrhythmia can be obtained with medication, the hamartomas frequently will regress (56). In rare cases, when control cannot be achieved with maximal medication, the patient may require surgical excision of the tumor (57). These tumors frequently are visible to the naked eye as pale colored areas of the myocardium and can be surgically excised.
Nonautomatic Focal Atrial Tachycardia
Nonautomatic focal atrial tachycardias (NAFAT) are the result of a small reentrant circuit within the atrium (i.e., micro-reentrant tachycardia) (58). This type of tachycardia, like other reentrant tachycardias, can be reproducibly induced with programmed atrial stimulation and can be terminated with adenosine. It commonly is located in the right atrium in patients with either normal or structurally diseased hearts (59). Although electrophysiologically distinct from AET, its management is similar, and the tachycardia focus is amenable to ablation.
Chaotic Atrial Tachycardia
Chaotic atrial tachycardia or multifocal atrial tachycardia is a type of atrial tachycardia with three or more foci, resulting in multiple P-wave morphologies on the ECG. It has been described in children with otherwise normal hearts as well as in patients with acute viral bronchiolitis or advanced pulmonary disease. Because of the rapid chaotic nature of the tachycardia, it can be difficult to distinguish from atrial fibrillation. This type of tachycardia can be difficult to treat and is generally refractory to pace termination, DC cardioversion, and adenosine. Because of the multiple foci, this type of tachycardia is not amenable to ablation. When long-term medication therapy is used, it is typically recommended to use Vaughn Williams class IA, IC, or III agents either alone or in combination (Table 22.3). As in atrial tachycardia, there is a high incidence of spontaneous resolution if it presents in the newborn period.
TABLE 22.3 Vaughan Williams Classification | |||||
|---|---|---|---|---|---|
|
Junctional Ectopic Tachycardia
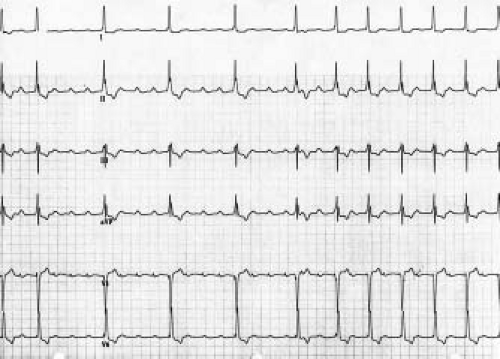
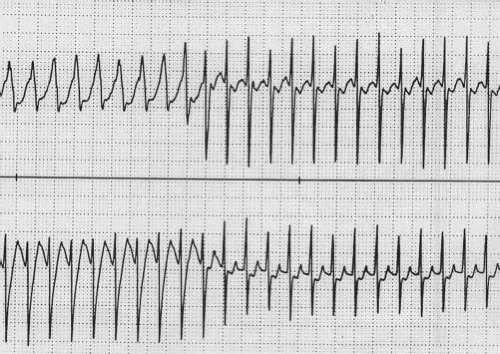
JET is an automatic focus tachycardia arising within or immediately adjacent to the AV junction. It is usually a narrow complex tachycardia with a QRS morphology identical or very similar to the QRS morphology in sinus rhythm (Fig. 22.10). It is classically described as a tachycardia that has no association between the P waves and the QRS complexes (dissociated) on the ECG. In reality, this tachycardia has a wide variety of junctional–atrial (J–A) relationships ranging from 1 to 1, to J–A Wenckebach, to no relationship. Retrograde P waves often are visualized in the terminal portion of the QRS. With adenosine, there may be loss of the retrograde conduction, resulting in dissociation of the P wave and the QRS. This tachycardia has two different subtypes, postoperative and familial, both of which present differently.
Postoperative JET occurs in up to 6% of pediatric patients who have undergone cardiac surgery and occurs more frequently when inotropic agents such as dopamine are administered (60). Congenital JET is one of the rarest forms of SVT in infants and also one of the most difficult to treat. Patients with congenital JET have a wide range of clinical presentations. The arrhythmia is frequently present at birth but may not be identified until months to years later. Patients with congenital JET who are younger than 6 months of age are more likely to have incessant JET and to have faster JET rates, and the mortality rate in this population is approximately 4% (61). With current medical, ablative, and device therapies, the majority of patients have a good outcome.
Atrial Flutter
Atrial flutter is an uncommon arrhythmia in the pediatric population. It typically occurs in fetuses, newborn infants, and occasionally postoperatively in congenital heart disease patients. Newborn infants generally present within the first 2 days of life with tachycardia and may have signs/symptoms of heart failure (62). The ECG in atrial flutter has continuous atrial activation creating a “saw-tooth” pattern (Fig. 22.11) with atrial rates between 300 and 600 bpm and
variable conduction to the ventricles. If the flutter waves cannot be distinguished easily on the ECG, adenosine can be administered to block the AV node thus revealing the underlying flutter waves. The treatment of choice is direct current-synchronized cardioversion at 0.5 to 1 J/kg. Atrial overdrive pacing also can be used if the atrial rate is slow, but this is more difficult to perform in newborns due to the extremely rapid rate. Newborns with atrial flutter typically have no structural heart disease and have no further problems after successful cardioversion. A 24-hour cardioscan should be placed after cardioversion to rule out an underlying reentrant tachycardia or atrial tachycardia that initiated the atrial flutter. In addition, an echocardiogram should be performed to rule out a structural abnormality causing atrial dilation. Generally, if there is no evidence of other arrhythmias or congenital heart disease, these infants do not require antiarrhythmic therapy and follow-up is not necessary (63). As atrial flutter is an unusual arrhythmia in young children and adolescents, a thorough evaluation to evaluate for congenital heart disease or underlying arrhythmia syndrome is warranted.
variable conduction to the ventricles. If the flutter waves cannot be distinguished easily on the ECG, adenosine can be administered to block the AV node thus revealing the underlying flutter waves. The treatment of choice is direct current-synchronized cardioversion at 0.5 to 1 J/kg. Atrial overdrive pacing also can be used if the atrial rate is slow, but this is more difficult to perform in newborns due to the extremely rapid rate. Newborns with atrial flutter typically have no structural heart disease and have no further problems after successful cardioversion. A 24-hour cardioscan should be placed after cardioversion to rule out an underlying reentrant tachycardia or atrial tachycardia that initiated the atrial flutter. In addition, an echocardiogram should be performed to rule out a structural abnormality causing atrial dilation. Generally, if there is no evidence of other arrhythmias or congenital heart disease, these infants do not require antiarrhythmic therapy and follow-up is not necessary (63). As atrial flutter is an unusual arrhythmia in young children and adolescents, a thorough evaluation to evaluate for congenital heart disease or underlying arrhythmia syndrome is warranted.
Atrial Fibrillation
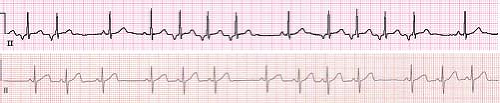
Atrial fibrillation is a very uncommon arrhythmia in the pediatric population. Atrial fibrillation presents as an irregularly irregular rhythm with most beats having the same QRS morphology seen in sinus rhythm (Fig. 22.12). The P waves are often difficult to visualize unless the patient is in coarse atrial fibrillation. Atrial fibrillation occurs in the context of WPW or following extensive surgery involving the atrium such as a total cavopulmonary connection or atrial switch operation (Mustard or Senning). Patients who present with atrial fibrillation without evidence of structural heart disease warrant evaluation for other arrhythmias, such as reentrant SVT, that degenerates into atrial fibrillation, underlying channelopathy, or drug use. It may be the initial presentation of hyperthyroidism, myocarditis, or digoxin toxicity. If no obvious cause is identified, the arrhythmia may be an isolated finding and is known as primary or lone atrial fibrillation, which usually begins in the late teenage years (64).
For the patient with chronic atrial fibrillation, it has been suggested that ablation of the AV node with implantation of a permanent ventricular pacemaker be performed. Because of the permanent loss of AV synchrony combined with the long-term need and dependence on a pacemaker as well as the need for chronic anticoagulation, this therapy should be reserved only for the most refractory and symptomatic cases of atrial fibrillation that have failed to respond to multiple medical therapies. Atrial fibrillation is typically treated with direct current cardioversion as antiarrhythmic medications are not particularly effective in acute conversion. However, because of the potential for thrombus formation in the left atrium due to the lack of organized contractions (particularly in patients with poor ventricular function), consideration should be given to anticoagulating patients for a period of 3 weeks prior to cardioversion in patients who have been in atrial fibrillation over 48 hours (65). Anticoagulation then should be continued for 4 weeks following cardioversion due to the atrial “stunning” that occurs. If a patient is decompensated with a rapid ventricular response or a decrease in cardiac function due to the arrhythmia, immediate cardioversion is warranted. A transesophageal echocardiogram should be considered to evaluate for a left atrial thrombus prior to cardioversion.
Intra-atrial Reentrant Tachycardia
Intra-atrial reentrant tachycardia (IART) generally occurs in patients who have extensive scarring in their atria either from previous cardiac surgery or a primary myocardial disease. This unique form of arrhythmia shares many characteristics similar to atrial flutter but tends to have slower rates ranging from 140 to 200 bpm. The ECG appearance is distinct in that there tends to be an isoelectric baseline between consecutive P waves, unlike atrial flutter, where constant atrial activity creates the saw-toothed pattern (Fig. 22.13). The P waves in IART may be very small and are often missed, particularly if they are buried within the QRS complex or the T wave. Because of the isoelectric baseline, IART is sometimes incorrectly interpreted as an ectopic atrial tachycardia or even sinus rhythm. As in atrial flutter, adenosine can be used to unmask IART, but it typically does not terminate it. Because IART often occurs in patients with underlying sinus node dysfunction (SND), a patient with SND and a heart rate faster than 80 to 100 bpm should be carefully examined for this arrhythmia. Another clue to the presence of underlying IART is new onset prolongation of the PR interval which is a result of the increased atrial rate creating decremental conduction in the AV node. Some utility has been found in placing prophylactic epicardial ablation lesions in strategic positions (the so-called maze or maze-Cox III procedure) to prevent IART, and atrial fibrillation in pediatric patients and adults undergoing surgical correction of a congenital heart defect. This procedure is particularly useful in patients who have had extensive atrial operations such as the Fontan procedure. Long linear cryoablation probes can be placed on
the surface of the heart and quickly form deep transmural lesions without the need to arrest the heart or place the patient on cardiopulmonary bypass. Lesions can also be performed endocardially or using catheter-based technologies. These lesions appear to have a relatively good long-term success even in the most complicated patients. For patients with atrial arrhythmias having a Fontan revision, there is up to a 76% recurrence without the placement of intraoperative ablation lesions (66). Physicians at many large centers routinely place ablation lesions (maze procedure) when doing a Fontan conversion. These lesions in combination with the placement of an atrial pacemaker result in a late recurrence of IART of only 13% to 22% (67,68).
the surface of the heart and quickly form deep transmural lesions without the need to arrest the heart or place the patient on cardiopulmonary bypass. Lesions can also be performed endocardially or using catheter-based technologies. These lesions appear to have a relatively good long-term success even in the most complicated patients. For patients with atrial arrhythmias having a Fontan revision, there is up to a 76% recurrence without the placement of intraoperative ablation lesions (66). Physicians at many large centers routinely place ablation lesions (maze procedure) when doing a Fontan conversion. These lesions in combination with the placement of an atrial pacemaker result in a late recurrence of IART of only 13% to 22% (67,68).
Treatment of Supraventricular Arrhythmias
Treatment of supraventricular arrhythmias depends on multiple factors including patient age, severity of symptoms, spontaneous termination, duration of episodes, presence of underlying cardiac defects, and frequency of episodes. Medical treatment typically is effective, but does not address the underlying cause. Catheter ablation, particularly in patients older than 5 years of age should be considered. Ablation indications are discussed in another chapter in this book (see Chapter 21).
Wide Complex Tachycardias
Wide complex tachycardias should be assumed to be VT until proven otherwise. However, there are many situations when a wide complex tachycardia is supraventricular in origin (SVT) (Fig. 22.14). The most common scenario is an SVT with aberrancy. With rapid onset of a tachycardia, either the left or right bundle branch can be
refractory and not able to conduct impulses, resulting in a wide complex tachycardia on the ECG. A left bundle branch block pattern of aberrancy is much more common in neonates, while a right bundle branch block pattern is more common in children and adolescents. Frequently, the bundle will accommodate the tachycardia, and the QRS complex narrows (Fig. 22.15). Other scenarios resulting in a wide complex SVT include the presence of an underlying bundle branch block or ventricular preexcitation or in an antidromic tachycardia circuit. Most wide complex tachycardias except for ventricular fibrillation are relatively regular tachycardias. When an irregular wide complex tachycardia is present, the possibility that it is atrial in origin and should be strongly considered.
refractory and not able to conduct impulses, resulting in a wide complex tachycardia on the ECG. A left bundle branch block pattern of aberrancy is much more common in neonates, while a right bundle branch block pattern is more common in children and adolescents. Frequently, the bundle will accommodate the tachycardia, and the QRS complex narrows (Fig. 22.15). Other scenarios resulting in a wide complex SVT include the presence of an underlying bundle branch block or ventricular preexcitation or in an antidromic tachycardia circuit. Most wide complex tachycardias except for ventricular fibrillation are relatively regular tachycardias. When an irregular wide complex tachycardia is present, the possibility that it is atrial in origin and should be strongly considered.
Ventricular Tachycardia
VT is defined as three or more premature ventricular contractions (PVCs) in a row at a rate faster than 120 bpm. Slower rates, within around 10% to 20% of the underlying sinus rate, are referred to as accelerated ventricular rhythms. Accelerated ventricular rhythms typically have no associated symptoms and have a benign prognosis making treatment of this condition unnecessary (Fig. 22.16) (69). A hallmark of VT is that the QRS complex is different than the sinus QRS complex but in pediatrics, there is no specific duration of the QRS complex required to meet criteria. In infants under 1 year of age, the QRS duration in VT can be as short as 0.06 seconds, and the rate can be as fast as 500 bpm (Fig. 22.17). It can also be relatively narrow in older children or have a sharp initial upstroke of the QRS when the focus of the tachycardia is in close proximity to or contained within the normal conduction system such as seen in fascicular VTs or bundle branch reentry. Most VTs, particularly in patients with structural heart disease, are reentrant in nature, although automatic foci also occur particularly in patients with structurally normal hearts. VT may be classified as monomorphic (all QRS complexes appear the same) or polymorphic (beat to beat variations in the QRS morphology). Polymorphic VT is less common in the pediatric population and typically has a less favorable prognosis than monomorphic VT (70).
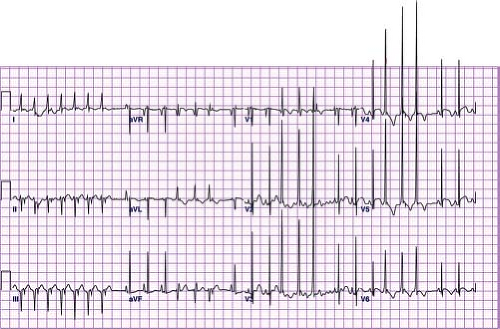 Figure 22.17 Ventricular tachycardia in a neonate. Note the relatively narrow complexes that are different from sinus beats and retrograde P waves in the initial portion of the tracing. |
In the normal pediatric population, spontaneous VT has an approximate incidence of 1/100,000 in the childhood years and may present any time between infancy and young adulthood (71). However, short episodes of VT can occur in up to 3% of otherwise healthy teenagers on routine Holter monitoring (72). In comparison to patients with structural heart disease or cardiomyopathy, patients with normal cardiac anatomy and function generally have a benign course. The presence of underlying heart disease, presence of symptoms, rapid rate of VT, and exacerbation of VT with exercise are risk factors for adverse events in nonischemic VT in children, and control of arrhythmias by ablation or antiarrhythmic medications may be indicated in this population (73). The chance of spontaneous resolution is higher when the onset occurs during the first year of life (resolution in about 90% of patients) compared with onset beyond the first year of life to 15 years of age (resolution in approximately 50%). The clinical profile is more favorable for patients with right-sided VT (resolution in 76% and symptoms in 25%) compared with patients with left-sided VT (resolution in only 37% and symptoms in 67%) (74).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree