Diseases of the Aorta
Heather L. Gornik
Mark A. Creager
Introduction
The aorta is the largest and most important blood vessel of the body, serving as the initial conduit for the perfusion of all organs and tissues. The spectrum of pathology of the aorta is wide reaching and includes congenital anomalies, degenerative abnormalities, atherosclerosis, and inflammation. Some aortic disorders present in childhood and young adulthood, whereas others generally occur among the elderly. In this chapter, we provide an overview of diseases of the aorta. Throughout the text, we emphasize recent advances in diagnostic modalities and endovascular and surgical technologies that have revolutionized the approach to the patient with aortic disease.
Anatomy of the Aorta
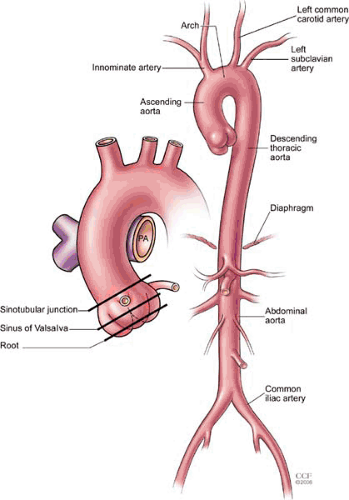
The aorta is the largest artery of the body, delivering oxygenated blood from the left ventricle to organs and tissues through its arterial branches. The aorta is divided into thoracic and abdominal segments based on the location relative to the diaphragm (Fig. 105.1). The thoracic aorta is further divided into the ascending aorta (containing the aortic root, the sinuses of Valsalva, and a tubular segment), the aortic arch (containing the great vessels), and the descending thoracic aorta. The aortic isthmus is a narrow region of the aorta located between the origin of the left subclavian artery and the ligamentum arteriosum. The thoracic aorta begins as an anterior structure, coursing superiorly to the right of the sternum, ultimately moving posteriorly into the mediastinum, and running along the left of the vertebral column. The descending thoracic aorta travels posterior to the esophagus and anterior to the vertebral column and gives rise to a number of branches, including small pericardial branches, bronchial and esophageal arteries, and the posterior intercostal arteries. The abdominal aorta begins at the diaphragm and ends at the aortoiliac bifurcation, giving off major visceral and mesenteric branches, including the celiac axis, superior and inferior- mesenteric arteries, and the paired renal arteries. The abdominal aorta also gives rise to paired lumbar branches, which supply the musculature of the back.
Congenital Anomalies of the Aorta
Although there are dozens of anatomic variations of the aorta and its major branches, only a small number of anomalies are associated with clinical consequences. The most common anomaly of the aortic arch is the so-called bovine variant, which is present in up to 10% to 20% individuals and is characterized by the origin of the left common carotid artery arising from the brachiocephalic (innominate) trunk. Other common aortic arch anomalies include a four-vessel arch with separate origins of the right common carotid and right subclavian arteries (2.5%), symmetric right and left brachiocephalic trunks forming a two-vessel aortic arch (1.2%), and origin of the left vertebral artery directly from a four-vessel aortic arch, typically between the ostia of the left common carotid and left subclavian arteries (2.4% to 5.8%) (1). None of these anomalies is typically associated with symptoms, although each is of potential importance in planning a catheter-based or open surgical procedure for another indication, such as occlusive disease of a carotid or subclavian vessel (2).
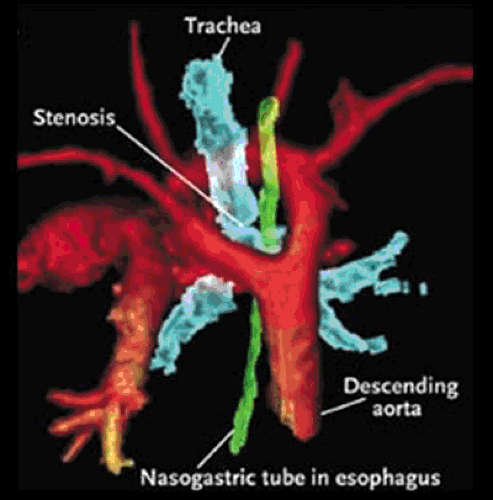
The rare aortic arch anomalies that are associated with clinical symptoms typically form a compressive vascular ring around the esophagus and the tracheal-bronchial tree, causing bronchospasm, stridor, chronic cough, or dysphagia. The most common vascular ring anomalies are double aortic arch and right-sided aortic arch with aberrant left subclavian artery and ligamentum arteriosum (Fig. 105.2) (3,4,5). Although it does not cause a true vascular ring, aberrant origin of the right subclavian artery distal to the left subclavian artery of a normal left-sided aortic arch is an anatomic aortic variant that is present in approximately 1% of the population (6). This anomaly is associated with marked dilation of the right subclavian artery,
often causing dysphagia as it passes retrograde to the esophagus toward the right arm. Such dysphagia due to a vascular ring is known as dysphagia lusoria (7). Aortic arch anomalies, particularly those with aberrance of one of the subclavian arteries, are also associated with an anatomic remnant of the persistent right aortic arch known as a Kommerell’s diverticulum (7,8,9). Kommerell’s diverticula may become aneurysmal.
often causing dysphagia as it passes retrograde to the esophagus toward the right arm. Such dysphagia due to a vascular ring is known as dysphagia lusoria (7). Aortic arch anomalies, particularly those with aberrance of one of the subclavian arteries, are also associated with an anatomic remnant of the persistent right aortic arch known as a Kommerell’s diverticulum (7,8,9). Kommerell’s diverticula may become aneurysmal.
Symptomatic aortic arch anomalies are extremely rare, and the index of suspicion of the clinician must be high to make the diagnosis. An aortic arch anomaly may be suspected in a patient with classical symptoms and an abnormal chest radiograph demonstrating enlargement or abnormal configuration of the aortic shadow. Arch anomalies may also be detected by transthoracic or transesophageal echocardiography (10). Diagnosis is confirmed by a noninvasive imaging study of the thoracic aorta and arch vessels, such as computed tomography (CT) or magnetic resonance angiography (MRA). Treatment of symptomatic vascular ring anomalies and aberrant subclavian arteries typically involves surgical correction. Procedural outcome depends on the age and comorbidities of the patient at the time of surgery. Outcomes of vascular ring surgery in the pediatric population are typically excellent, and small case series of successful surgery in adults have also been reported (3,4,5,11). Aneurysms of anomalous subclavian arteries or of Kommerell’s diverticula may spontaneously rupture or dissect, and prophylactic surgical correction is generally recommended, even in the absence of symptoms (9,12,13).
Aortic Aneurysms
The term aortic aneurysm, derived from the Greek word aneurysmos for “dilation,” refers to enlargement of the aorta beyond its normal diameter. A segment of the aorta is called aneurysmal if its maximal diameter is greater than 1.5 times that of the adjacent proximal normal segment. As general guidelines, the normal diameter of the thoracic aorta is approximately 2.5 cm at the aortic annulus, 3 cm at the tubular ascending portion, 2.5 cm at the descending thoracic aorta, and 2 cm in the infrarenal abdominal aorta (14,15). Aortic dimension varies by body surface area, age, and gender, with men typically having larger aortic dimensions than women (15). An
aneurysm is described as fusiform if it symmetrically involves the entire circumference of the aorta. In contrast, a saccular aneurysm involves a focal outpouching of an area of the vessel wall. Aortic aneurysm must also be differentiated from aortic pseudoaneurysm, a contained rupture of the vessel into the adventitial space. Aortic pseudoaneurysms are typically posttraumatic (Chapter 38) or due to penetrating atherosclerotic ulcer or infectious aortitis. The epidemiology, pathophysiology, and management of aortic aneurysm are highly dependent on the anatomic location of the lesion, as discussed later. For aortic aneurysms at all locations, the objective of management is to avoid the potentially lethal complications of aortic rupture or dissection.
aneurysm is described as fusiform if it symmetrically involves the entire circumference of the aorta. In contrast, a saccular aneurysm involves a focal outpouching of an area of the vessel wall. Aortic aneurysm must also be differentiated from aortic pseudoaneurysm, a contained rupture of the vessel into the adventitial space. Aortic pseudoaneurysms are typically posttraumatic (Chapter 38) or due to penetrating atherosclerotic ulcer or infectious aortitis. The epidemiology, pathophysiology, and management of aortic aneurysm are highly dependent on the anatomic location of the lesion, as discussed later. For aortic aneurysms at all locations, the objective of management is to avoid the potentially lethal complications of aortic rupture or dissection.
Thoracic Aortic Aneurysms
Thoracic aortic aneurysms (TAAs) encompass all aneurysms that involve the aorta from the level of the aortic root to the crura of the diaphragm. Thoracic aortic aneurysms are categorized by whether they involve the aortic root and/or ascending aorta, aortic arch, or descending thoracic aorta. The subset of TAAs that extend directly into the abdominal cavity are called thoracoabdominal aneurysms (TAAAs). Approximately 60% of TAAs involve the aortic root or the ascending thoracic aorta, 40% involve the descending thoracic aorta, and 10% involve the aortic arch (16). Thoracoabdominal aneurysms are relatively rare (10%) (16).
Pathophysiology
The most common causes of TAA are listed in Table 105.1. The final common pathway and underlying histopathology of most ascending aortic aneurysms is degeneration of the medial layer of the aortic wall known as cystic medial necrosis. Thus, the risk factors for the development of ascending aortic aneurysm are primarily those conditions that are associated with cystic medial necrosis of the ascending aorta. Histologically, there is loss of elastin fibers and smooth muscle cells within the medial layer as well as accumulation of ground substance, leading to cystic-appearing spaces within the media (17,18). Cystic medial necrosis weakens the aortic wall and is an important underlying factor in both aneurysmal dilation of the aorta and aortic dissection. Cystic medial necrosis has been reported as a common pathologic finding among ascending aortic aneurysms related to Marfan syndrome, congenital bicuspid aortic valves, and in the familial TAA syndromes among others (18,19). Marfan syndrome is an autosomal dominant disorder associated with mutations in the genes for fibrillin-1, a key component of myofibrils of elastin. The type IV variant of Ehlers-Danlos is an autosomal disorder due to mutations in genes for type III procollagen (20). Three specific loci for distinct familial TAA and dissection syndromes have been reported (21,22,23). Cystic medial necrosis may also occur in the setting of longstanding hypertension, particularly in elderly patients (24). A variant of cystic medial necrosis, annuloaortic ectasia, or dilation of the aorta at the level of the annulus, may cause isolated aortic insufficiency in association with TAA. Annuloaortic ectasia may occur in association with Marfan syndrome or other genetic disorders or may occur as an idiopathic variant. The cause of cystic medial necrosis is not known, although it appears that genetically programmed premature apoptosis of smooth muscle cells in media may play a role (18).
TABLE 105.1 Etiology of Thoracic Aortic Aneurysms | |
|---|---|
|
Aneurysms of the descending thoracic aorta and thoracoabdominal aneurysms are degenerative and often have pathologic characteristics of atherosclerosis. These include distortion of the arterial intima and media leading to weakening of the aortic wall and subsequent aneurysmal dilation. Recent clinical investigation, however, has challenged the notion that descending TAAs are causally related to systemic atherosclerosis (25). In addition to atherosclerosis and cystic medial necrosis, TAA may occur as a consequence of chronic aortic dissection or in response to the inflammation of a large-vessel vasculitis. Infectious, or mycotic, TAAs are related to chronic syphilis or caused by select bacterial or mycobacterial infections. Mycotic
aneurysms are often saccular in appearance and are associated with a high rate of rupture or contained rupture with pseudoaneurysm formation (26,27). Sinus of Valsalva aneurysm is a rare anomaly that involves aneurysmal dilation of one of the coronary sinuses, typically the right coronary sinus (28). Sinus of Valsalva aneurysms are usually congenital, but they may also occur as a complication of aortic trauma (i.e., cardiac catheterization with aortic injury), infection, or inflammation. These lesions are prone to spontaneous rupture, including fistulization into a cardiac chamber, particularly the right ventricle (28).
aneurysms are often saccular in appearance and are associated with a high rate of rupture or contained rupture with pseudoaneurysm formation (26,27). Sinus of Valsalva aneurysm is a rare anomaly that involves aneurysmal dilation of one of the coronary sinuses, typically the right coronary sinus (28). Sinus of Valsalva aneurysms are usually congenital, but they may also occur as a complication of aortic trauma (i.e., cardiac catheterization with aortic injury), infection, or inflammation. These lesions are prone to spontaneous rupture, including fistulization into a cardiac chamber, particularly the right ventricle (28).
Epidemiology and Prognosis
The estimated incidence of TAA is between 5.9 and 10.4 new aneurysms per 100,000 person-years (29,30). Among patients with degenerative (non–Marfan associated) TAA, diagnosis is most commonly made in the sixth or seventh decades of life, with women typically presenting up to a decade later than men (29,30). Natural history studies have estimated that the average expansion of degenerative TAA is 0.1 cm/year, with descending or thoracoabdominal aneurysms growing at a more rapid rate (0.19 cm/year) than aneurysms of the ascending aorta or aortic arch (0.07 cm/year) (31). Marfan disease–associated TAA and chronic dissecting aneurysms also grow at an increased rate (31). According to the law of Laplace, wall stress is directly proportional to the radius of the vessel. As aneurysms enlarge, there is increased wall stress, accelerated rate of expansion, and an increased risk of dissection or rupture. For TAAs less than 4 cm in diameter, the mean rate of rupture or dissection is less than 3%/year (31). For aneurysms larger than 6 cm in diameter, the estimated rate of rupture or dissection is 6.9%/year with overall mortality of 11.8%/year (31). Whereas aortic dissection and rupture are the leading causes of death among patients with degenerative TAA, cardiovascular events account for one fourth of deaths, which likely reflects the age of the population and extensive systemic burden of atherosclerosis (30,31). In addition to increasing aneurysm size, risk factors for aortic dissection, rupture, or death among patients with TAA include Marfan syndrome, history of a prior cerebrovascular event, and female gender (31). Pregnancy is also strongly associated with adverse outcomes among women with TAA due to Marfan syndrome or bicuspid aortic valve, and there are case reports of rapid aneurysmal expansion, rupture, and dissection during pregnancy (32,33).
Clinical Presentation and Diagnosis
The role of the history and physical examination in the diagnosis of TAA is limited. Most patients with TAA are asymptomatic and are diagnosed on the basis of a noninvasive imaging study that has been obtained for another indication. When present, symptoms are generally due to direct compression of surrounding intrathoracic structures, such as the superior vena cava (facial swelling), esophagus (dysphagia), trachea or bronchi (wheezing, dyspnea, chronic cough), or recurrent laryngeal nerve (hoarseness) (34). Chest or back discomfort is also a common symptom among patients with large TAA, particularly in association with rapid expansion of the aneurysm.
Among asymptomatic patients, a family history of Marfan syndrome or type IV Ehlers-Danlos syndrome should alert the clinician to the possibility of occult TAA. A dedicated imaging study of the aorta should be performed in these patients, particularly in the presence of physical findings suggestive of a genetic disorder. Physical findings of TAA, such as a visible bulge or a parasternal heave, occur rarely. Diagnostic clues for TAA relate to the underlying etiology, including signs of Marfan syndrome (i.e., tall stature, arachnodactyly, pectus deformity, and ectopia lentis). Cardiac murmurs of aortic insufficiency or aortic stenosis may be appreciated among patients with ascending TAA, particularly those with bicuspid aortic valve, who may also have a systolic ejection click. Multiple imaging modalities are used to diagnose TAA. Chest radiography, though too insensitive to be used as the sole diagnostic modality, may demonstrate widening of the aortic knob or mediastinal silhouette. In general, if there is any clinical suspicion of TAA, a dedicated imaging study of the aorta should be obtained. Magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) are both excellent imaging modalities for the diagnosis and surveillance of TAA (35). The latest generation of multidetector CT scanners allow for excellent spatial resolution and sizing of the aneurysm as well as visualization of branch vessels and vascular calcification. CTA also readily diagnoses complications associated with aortic aneurysm, particularly aortic rupture or dissection. Cross-sectional CTA images can be used to generate three-dimensional reconstructions that mimic conventional aortography. CT angiography is the imaging modality of choice for follow-up after surgical or endovascular repair of a TAA. To prevent excessive interreader variability, orthogonal measurements of maximal aortic diameter, as measured from outer wall to outer wall of the aorta, should be taken using standardized protocols (36). Magnetic resonance angiography with gadolinium enhancement and a breath-hold is superior to time-of-flight magnetic resonance imaging for the sizing and characterization of TAA and also allows for the generation of angiographic projections in many planes of view (Fig. 105.3). Transthoracic echocardiography (TTE) is an excellent imaging modality for diagnosis of TAA located at or near the aortic root. Echocardiography is also able to diagnose cardiac pathologies associated with TAA, particularly bicuspid aortic valve. Transthoracic echocardiography may fail to detect aortic dilation in the tubular portion of the ascending aorta and is unable to adequately visualize the aortic arch and the descending thoracic aorta. Transesophageal echocardiography (TEE) is able to visualize the entire thoracic aorta, with the exception of a segment of the distal ascending aorta, which is obscured by tracheal shadowing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree