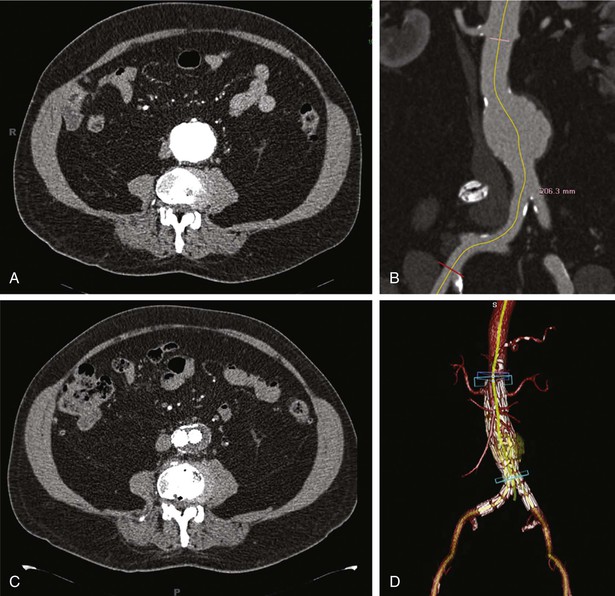
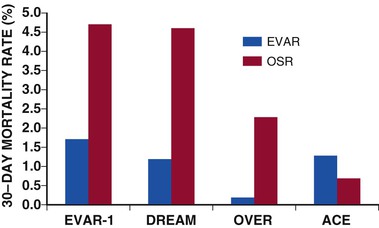
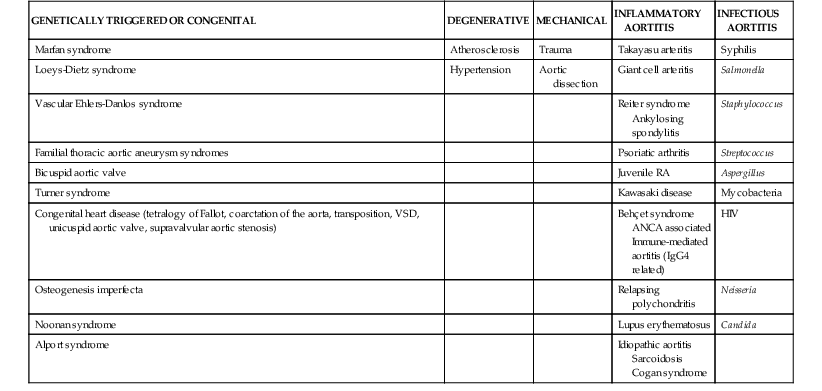
Alan C. Braverman The aorta, the largest artery in the body, is divided anatomically into thoracic and abdominal components. The thoracic aorta is subdivided into the ascending, arch, and descending segments, and the abdominal aorta, into the suprarenal and infrarenal segments. The ascending aorta has two distinct portions. The aortic root begins at the aortic valve and extends to the sinotubular junction. The aortic root supports the bases of the three aortic valve leaflets, which bulge outward into the sinuses of Valsalva during systole. The origins of the right and left coronary arteries arise from the sinuses of Valsalva. The upper portion of the ascending aorta begins at the sinotubular junction and rises to join the aortic arch. The proximal portion of the ascending aorta lies within the pericardial cavity, anterior to the pulmonary artery bifurcation. The aortic arch gives rise to the innominate artery, the left common carotid artery, and the left subclavian artery. The descending thoracic aorta begins distal to the left subclavian artery. The point at which the aortic arch joins the descending aorta, denoted the aortic isthmus, is marked by the location of the ligamentum arteriosum. The aortic isthmus is vulnerable to deceleration trauma because at this site the ascending aorta and arch become relatively fixed to the thoracic cage. The descending aorta gives rise to posterior paired intercostal arteries at each level of the spine. Distally, the thoracic aorta passes through the diaphragm and becomes the abdominal aorta. The abdominal aorta gives rise to the celiac artery and the superior mesenteric artery anteriorly, followed by the posterolateral origins of the right and left renal arteries. This segment of the aorta is called the suprarenal or visceral segment. The infrarenal aorta lies anterior to the lumbar spine, where paired lumbar artery braches arise posteriorly. The aorta ends by bifurcation into common iliac arteries. The aorta transmits pulsatile arterial blood pressure to all points in the arterial tree, a function that depends on its properties as an elastic conduit. The biomechanical properties of the aorta, including resilience to cyclical deformation, are attributable to elastin and collagen in the media and adventitia. The aortic wall pressure-diameter relationship is nonlinear; a more distensible component is demonstrated at lower pressures and a stiffer component at higher pressures, with the transition from distensible to stiff behavior occurring at pressures higher than 80 mm Hg. The pressure-diameter curve of the aorta becomes less steep with increasing age (i.e., the aorta stiffens and aortic diameter increases). Potential explanations for this change include (1) an increase in the collagen-to-elastin ratio because of a decrease in elastin and an increase in collagen, (2) changes in the aortic wall with progressively disordered medial elastic fibers and lamellae displaying thinning and fragmentation, (3) an increase in aortic wall thickness with deposition of collagen and other extracellular matrix macromolecules and calcification of elastic fibers, and (4) arteriosclerotic changes leading to wall stiffening. The only location in which the aorta can normally be palpated is in the midabdominal region, where in some individuals (depending on body habitus) it may be detected by deep palpation adjacent to the spine. Plain radiography is insensitive in evaluating the thoracic and abdominal aorta, but much more diagnostic detail regarding the aorta can be obtained with imaging modalities such as ultrasound (including echocardiography), computed tomography (CT), magnetic resonance imaging (MRI), and less frequently, aortography. The term aortic aneurysm refers to a pathologic segment of aortic dilation that has a propensity to expand and rupture. The extent of aortic dilation required to be considered aneurysmal is debated, but one criterion is an increase in diameter of at least 50% greater than expected for the same aortic segment in unaffected individuals of the same age and sex. Aortic aneurysms are usually described in terms of their size, location, morphology, and cause. Size criteria are focused on cross-sectional diameter as measured on imaging studies. Aortic aneurysms are either fusiform or saccular. Fusiform aneurysms, the more common type, are symmetrically dilated with involvement of the entire aortic circumference. Saccular aneurysms exhibit localized dilation involving only a portion of the aortic wall circumference, where they appear as a focal outpouching. These lesions represent “true” aneurysms in that the aortic wall is intact but dilated and all layers of the aortic structure are involved. In contrast, pseudoaneurysms (false aneurysms) represent lesions in which bleeding has occurred through the aortic wall and resulted in a contained periaortic hematoma in continuity with the aortic lumen. Pseudoaneurysms may result from trauma or contained rupture of an aortic aneurysm, dissection, or penetrating ulcer. AAAs are defined by an increase in size of the abdominal aorta to greater than 3.0 cm in diameter.1 AAAs occur in 3% to 9% of men older than 50 years and are the most common form of aortic aneurysms. Most AAAs (>80%) arise in the infrarenal aorta (Fig. 57-1), but up to 10% may involve the pararenal or visceral aorta and some extend into the thoracoabdominal segment. AAAs are approximately five times more prevalent in men than in women, and their incidence is strongly associated with age, with most occurring in those older than 60 years.2 AAAs are also strongly associated with cigarette smoking, with current and former smokers having a fivefold increase in risk in comparison to nonsmokers. Additional risk factors include emphysema, hypertension, and hyperlipidemia. Up to 20% of patients with AAAs describe a family history of aortic aneurysms, thus suggesting the contribution of a heritable component. AAA formation is associated with chronic aortic wall inflammation, increased local expression of proteinases, and degradation of structural connective tissue proteins. Aneurysmal dilation and rupture result from mechanical failure of medial elastin and adventitial collagen. Inflammatory cells commonly infiltrate the aortic wall. In some cases, patients with “inflammatory AAAs” exhibit extension of this process to the periaortic retroperitoneal tissues. Matrix-degrading enzymes released by inflammatory cells lead to medial degeneration and play a role in dilation and rupture. Inflammatory cells may enter the media in response to signals elaborated by medial SMCs as a result of hemodynamic stress, ischemia, autoimmune processes, or extension of intimal atherosclerosis. Proinflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6, and interferon-gamma, may play a role. Although a response to both foreign antigens and microbial infection has been postulated in the development of AAAs, evidence shows that the chronic inflammation in aneurysm tissue also exhibits features of an autoimmune response. Destruction of medial elastin and a marked decrease in the concentration of elastin are consistent features of AAAs. Experimental studies have demonstrated that damage to the elastic lamellae leads to aneurysmal dilation, and elastolytic proteinases may play a critical role. The tensile strength of the aortic wall results principally from interstitial collagen, and AAAs are generally associated with increased collagen content. Enzymes that initiate cleavage of interstitial collagen may contribute to rapid aneurysm expansion and rupture. The most prominent elastin- and collagen-degrading enzymes produced in human AAA tissue are matrix metalloproteinases (MMPs). MMPs degrade a broad range of matrix proteins, with four MMPs exhibiting activity against elastin (MMP-2, MMP-7, MMP-9, and MMP-12). At least three MMPs initiate the degradation of intact fibrillar collagen (MMP-1, MMP-8, and MMP-13). MMP activity is closely regulated at the level of gene transcription, as well as by proteases and reactive oxygen species that mediate extracellular activation and by interaction with secreted tissue inhibitors of metalloproteinases (TIMPs) and other proteinase scavengers. MMP-9 appears to be especially important in human and experimental AAAs; MMP-9 expression is markedly elevated in aneurysm tissue, and AAAs are suppressed in mice lacking expression of MMP-9. Moreover, treatment of experimental animals with tetracyclines and other MMP inhibitors has consistently suppressed aneurysm development. Other experimental interventions found to suppress AAAs, such as treatment with statins and anti-inflammatory agents, have decreased MMP-9 in aortic tissue. These observations have led to the potential use of doxycycline and other MMP inhibitors to suppress the progression of aneurysms in patients with small AAAs.3 The natural history of AAAs involves a balance between degradative and reparative processes. Because vascular SMCs normally produce elastin and collagen during aortic development and SMCs predominate within the elastic media, they may mediate repair of connective tissue within AAAs. Depletion of medial SMCs characterizes AAAs. Mechanisms underlying the loss of SMCs in AAAs include apoptosis, which may be initiated by medial ischemia, signaling molecules, or cellular immune responses. Medial SMC ischemia has been considered another factor involved in AAA degeneration because in the absence of vasa vasorum, the nutrient supply to the media depends on diffusion from the aortic lumen—which may be jeopardized by intimal thickening and atherosclerotic plaque. AAAs develop insidiously over a period of several years and rarely cause symptoms in the absence of distal thromboembolism, rapid expansion, or rupture. Although large AAAs are at substantial risk of rupturing, the vast majority of AAAs are small. Most AAAs are detected by screening studies or as an incidental finding on imaging studies performed for another purpose. Physical examination is insensitive in detecting AAAs, but abdominal palpation may reveal a pulsatile epigastric or periumbilical mass, particularly in thin patients with large aneurysms. Only 30% to 40% of AAAs are noted on physical examination, although aneurysms larger than 5 cm are detected in approximately 75% of patients, depending on body habitus.2 The mural thrombi associated with AAAs may lead to thromboembolism, which may be the initial symptom in 2% to 5% of patients. Evaluation for AAA in patients with other vascular diseases is important—an AAA is present in up to 85% of patients with a femoral artery aneurysm and in approximately 60% of patients with a popliteal artery aneurysm.2 Abdominal ultrasound can detect AAAs with high accuracy and a sensitivity and specificity of almost 100% and is preferred over CT in screening for AAAs because it is inexpensive and noninvasive and avoids exposure to radiation and contrast agents.2 Ultrasound also permits serial measurement of AAA size during the follow-up of patients with small AAAs. Because ultrasound-derived measurements of AAA diameter are less accurate than those obtained by CT or MRI, many recommend the use of ultrasound for follow-up of small AAAs and use CT or MRI for larger AAAs. Abdominal CT is extremely accurate in both detection of AAAs and measurement of aneurysm diameter (Fig. 57-1). When combined with radiographic contrast enhancement, thin-slice techniques, and three-dimensional reconstructions with measurements obtained perpendicular to the center line of the aorta, CT angiography (CTA) is more accurate than ultrasound. CTA is especially useful in demonstrating the extent of aneurysmal disease; the relationship of the AAA to the renal, visceral, and iliac arteries; and patterns of mural thrombus, calcification, or coexisting occlusive atherosclerosis, which might influence AAA repair. Three-dimensional reconstructions, including multiplanar and volume-rendering techniques, enhance visualization of the AAA before endovascular aneurysm repair (EVAR) (Fig. 57-1). CT is also preferred for the assessment of AAA variants, such as inflammatory AAAs and mycotic aneurysms. Magnetic resonance angiography (MRA) also has high accuracy in detecting AAAs, measuring aneurysm diameter, and planning treatment. MRA avoids exposure to radiation and iodine-based contrast material. CT is the preferred imaging modality, however, for evaluation of AAAs in most institutions. CTA has superseded aortography in the evaluation and management of AAAs. In patients undergoing EVAR, aortography is an initial step in the operative procedure. It is also used in subsequent interventions following AAA stent-graft repair, such as embolization of the lumbar or iliac artery branches. The characteristics of AAAs include an enlarged abdominal aortic segment marked by calcification. The aortic lumen may or may not appear enlarged because of the presence of mural thrombus. Screening for AAAs with ultrasound, coupled with repair of AAAs above a given size threshold, has reduced AAA-related deaths.1,2 The overall incidence of screening-detected AAAs ranges from 1 per 1000 in adults younger than 60 years to 7 per 1000 in those in their mid-60s, but it may be as high as 10% in those with risk factors such as older age, male sex, smoking, family history, history of other aneurysms, hypertension, atherosclerotic diseases, and hypercholesterolemia. In asymptomatic U.S. veterans 50 to 79 years of age, 66% of AAAs identified by screening were smaller than 4.0 cm.2 Aneurysm screening is associated with a 50% reduction in rupture and a 50% decrease in aneurysm-related mortality.1,2 Even though AAA screening is cost-effective in men 65 to 74 years of age, the cost-effectiveness of screening for AAAs in women remains controversial,1 and routine screening of women has not demonstrated a survival benefit.2 Although women have a lower prevalence of AAAs than men do, AAAs occur about 10 years later in women, and rates of rupture and mortality from rupture are both higher. In 2005, the U.S. Preventive Services Task Force recommended a one-time ultrasound screening for AAAs in men 65 to 75 years of age with a history of smoking.1,2 The Society for Vascular Surgery recommends a one-time screening for AAAs in all men older than 65 years or as early as 55 years in men and women with a family history of AAAs.2 Several genetic disorders are associated with thoracic aortic aneurysms (TAAs), including Marfan syndrome (MFS), Loeys-Dietz syndrome (LDS), and vascular Ehlers-Danlos syndrome (vEDS), but less commonly with aneurysms of the abdominal aorta. (See the section Thoracic Aortic Aneurysms.) Up to 20% of patients with an infrarenal AAA have a family history of AAAs, thus suggesting an inherited component. Several genetic variants appear to be linked with AAAs through analysis of single-nucleotide polymorphisms (SNPs) in large populations. A common sequence variant on chromosome 9p21 (rs10757278-G) is associated with a 31% increased risk for AAAs, as well as increased risk for intracranial aneurysms.4 Broader use of genome-wide screening may identify additional genetic factors associated with AAAs. The natural history of AAAs is gradual expansion over a period of years and eventual rupture. The average rate of expansion of AAAs between 3 and 5.5 cm ranges from 0.2 to 0.3 cm/year.1 Not all AAAs follow a linear or consistent rate of expansion. Some patients may have stable AAAs that grow slowly for years, whereas others may have a stable AAA size for many years, followed by a sudden increase within a short period. Although the size of the aneurysm is most important in predicting rupture, size alone may not predict risk for rupture. Wall thickness, intraluminal thrombus thickness, and peak wall stress may all contribute.2 The largest aneurysms have the highest risk for rupture, with the 1-year risk for rupture estimated to be 10% to 20% for AAAs 6.0 to 7.0 cm in diameter; 20% to 40% for AAAs 7.0 to 8.0 cm; and 30% to 50% for AAAs larger than 8.0 cm. The 5-year risk for rupture is approximately 5% for AAAs 3.0 to 4.0 cm in diameter, 10% to 20% for AAAs 4.0 to 5.5 cm, 30% to 40% for AAAs 5.5 to 6.0 cm, and higher than 80% for AAAs larger than 7.0 cm.2 Symptoms directly attributable to AAAs are usually related to overt rupture of the aneurysm or rapid expansion and impending rupture. Rupture of AAAs into the peritoneal cavity results in acute hemorrhage, severe abdominal pain, and hypotension as a consequence of exsanguination. Rupture into the retroperitoneum may result in a temporarily contained periaortic hematoma, with severe abdominal or back pain that may radiate to the flank or groin. A tender pulsatile abdominal or flank mass is often present, along with hypotension and/or syncope. Approximately 30% to 50% of patients with ruptured AAAs die before hospitalization, and an additional 30% to 40% die after reaching a hospital but before treatment.2 The operative mortality rate for open surgical repair (OSR) after AAA rupture is 40% to 50%, but it may be lower with EVAR.1,2 Hemodynamically stable patients with symptomatic but apparently unruptured AAAs should undergo CT to determine whether rupture has occurred. Because emergency repair entails a fourfold to fivefold higher mortality rate, in the absence of rupture, it may be prudent in certain cases to delay surgical repair for 4 to 24 hours until optimal conditions can be achieved, with the patient being closely monitored.2 Patients with small AAAs can be observed safely with imaging surveillance and little risk for rupture. In general, AAA repair is reserved for asymptomatic aneurysms at least 5.0 to 5.5 cm in diameter.1,2 Symptomatic aneurysms and those with rapid growth (>1 cm/year) require vascular surgical consultation.1 In patients with AAAs larger than 4.5 cm, CT is preferred over ultrasound for more accurate measurement of AAA size. Surveillance of aneurysms until the diameter exceeds 5.5 cm is associated with a low rate of rupture (≈1% per year).2 The Society of Vascular Surgery guidelines suggest the following surveillance strategy for AAAs of various size: 2.6 to 2.9 cm, imaging at 5 years; 3.0 to 3.4 cm, imaging every 3 years; 3.5 to 4.4 cm, imaging at 12 months; and 4.5 to 5.4 cm, imaging every 6 months.2 Uncertainty exists regarding the ultimate therapy for AAAs between 4.5 and 5.4 cm, and recommendations must be individualized. Young, healthy patients—especially women—with AAAs between 5 and 5.4 cm may benefit from early repair.2 Several steps are recommended for patients with AAAs to help minimize the risk for expansion of the aneurysm and improve overall health. Smoking cessation is important inasmuch as strong evidence has linked ongoing tobacco use with more rapid rates of AAA expansion and rupture. Statin use can be recommended for almost all patients with AAAs based on the presence of coexisting atherosclerotic disease, and although randomized data are lacking, these medications may suppress AAA growth.3 Even though no available data have shown a benefit of angiotensin-converting enzyme (ACE) inhibitors on AAA expansion, they do demonstrate benefit in patients with vascular disease and should be considered.1,2 Currently, ACE inhibitors are being studied as part of two randomized controlled trials involving small AAAs. Patients with small AAAs should be encouraged to exercise regularly because moderate physical activity does not adversely influence the risk for rupture and may even limit the rate of AAA growth. The potential use of pharmacologic therapies to suppress the growth rate of small AAAs and to reduce the need for surgical repair is of great interest.2,3 One of the earliest approaches suggested was the use of beta-adrenergic receptor–blocking agents (beta blockers) as a strategy to diminish hemodynamic stress. Although successful in animal models of AAAs, two large clinical trials demonstrated no benefit of propranolol treatment in patients with small AAAs.2 Suppression of specific proteinases involved in degradation of the extracellular matrix is another approach. Treatment with doxycycline has suppressed or prevented AAAs in animal models in association with MMP inhibition, particularly inhibition of MMP-9.3 Doxycycline is well tolerated by patients with small AAAs, in whom it also appears to decrease MMP activity in aneurysmal aortic tissue and in the circulation. Further investigation is needed to determine whether doxycycline treatment can reduce the rate of AAA expansion. A third experimental approach is the use of ACE inhibitors or angiotensin receptor–blocking agents (ARBs), such as losartan, to modify the metabolism of connective tissue in the aortic wall. An increased risk for AAA rupture has been reported in individuals who stopped taking ACE inhibitors in the months before rupture.2 The decision to undergo elective repair of an asymptomatic AAA depends on life expectancy and the estimated risk for rupture, balanced against the estimated risks associated with AAA repair. Factors significantly influencing operative morbidity and mortality include coronary artery disease (the leading cause of early and late mortality after AAA repair), chronic kidney disease, chronic obstructive pulmonary disease (COPD), and diabetes mellitus.2 Thus further evaluation for these conditions is warranted before elective AAA repair, along with optimization of preoperative status. Because many patients with AAAs have underlying coronary artery disease and because postoperative myocardial infarction (MI) poses a substantial risk for death or later cardiovascular events, special attention is directed toward coronary disease before elective AAA repair. Current guidelines state that in the absence of an active cardiac condition, further noninvasive testing is indicated only if it will change management. Some patients benefit from preoperative evaluation for coronary ischemia and treatment (see Chapter 80). Perioperative medical management to reduce cardiac risk in patients undergoing AAA repair may include appropriate administration of beta blockers, statins, and/or aspirin, in accordance with each individual patient’s risk factors and medical findings.2 Surgical treatment of AAAs can be performed by one of two general approaches: OSR or EVAR. Selection of the approach depends on the individual anatomy and on secondary factors such as patient age and estimated risks associated with anesthesia and surgery, with most patients undergoing EVAR.1,2 For OSR of infrarenal AAAs, the abdominal aorta may be approached through either a transperitoneal or a left retroperitoneal exposure. A tube or bifurcated prosthetic graft is attached with suture directly to the proximal aorta, followed by sutured anastomosis to either the distal aorta (tube graft) or the common iliac arteries (bifurcation graft). Following restoration of lower extremity flow through the aortic graft, the aneurysm sac is sewn together to prevent contact between the prosthetic graft and the gastrointestinal tract. The operative mortality rate for OSR ranges from 1% to 4% in reports from single-institution centers of excellence, whereas mortality rates in state or national data bases range from 4% to 8%.2 Operative complication rates range from 10% to 30%, with morbidity being related to cardiac, pulmonary, and renal complications and colonic ischemia. Because outcomes with OSR are related to hospital and surgeon volumes, there is a trend to recommend that OSR for AAAs be performed at centers with demonstrable operative mortality rates lower than 5%. Late complications develop in as many as 15% to 30% of patients in long-term follow-up after OSR for AAAs. Such complications include problems related to the abdominal incision, para-anastomotic aneurysms (including false aneurysms secondary to disruption of the suture line and true aneurysms secondary to proximal aortic degeneration), graft infection, graft-enteric erosions or fistula, and graft limb occlusions with lower extremity ischemia. Late aneurysm formation at anastomotic sites after OSR is uncommon and has been reported in 1%, 5%, and 20% of patients, respectively, at 5, 10, and 20 years postoperatively.2 Annual clinical follow-up with CT at 5-year intervals is generally recommended after open AAA repair. In patients with suitable anatomy, EVAR offers a less invasive alternative to OSR. EVAR requires adequate nonaneurysmal proximal and distal attachment sites, and proximal attachment of the graft may be achieved via infrarenal or suprarenal fixation.2 Several endografts have been approved by the Food and Drug Administration, each with its own unique design and method of fixation to the aortic wall.1,2 Randomized prospective trials comparing EVAR with OSR for asymptomatic infrarenal AAAs have demonstrated a lower 30-day mortality rate with EVAR than with OSR1,2 (Fig. 57-2), and a meta-analysis of these trials also reported a lower perioperative and intermediate survival benefit for the EVAR group.5 A significantly higher number of repeated interventions, however, occurred in the EVAR group.5 Large data bases have reported low mortality rates with EVAR, and when a high-risk cohort from the Veterans Affairs National Quality Improvement Program was analyzed, risk for mortality was found to be lower with elective EVAR than with OSR.2 At long-term (≈5-year) follow-up, however, AAA-related or all-cause mortality did not differ significantly between EVAR and OSR.6 Patients with ruptured AAAs may also benefit significantly from EVAR. In evaluating 27,750 patients discharged from the hospital after ruptured AAAs, EVAR was associated with lower overall in-hospital mortality than OSR was (32% to 41%, P <0.0001).7 Data on 1037 patients treated by EVAR and 763 treated by OSR were collected from 13 centers. The overall 30-day mortality in all patients undergoing EVAR was 21%. In centers performing EVAR for all ruptured infrarenal AAAs, the 30-day mortality rate was 24%. When EVAR was compared with OSR from 1998 to 2009, EVAR was found to be associated with a lower 30-day mortality rate than OSR was, 16% versus 37%.8 With appropriate patient selection and accurate graft deployment, low perioperative mortality (1% to 2%) and complication (10% to 15%) rates can be achieved with EVAR for elective AAA repair.2 These results have led to increased application of EVAR in patients with AAAs and appropriate anatomy. Currently, the options of EVAR and OSR, with their advantages and disadvantages, are considered in “medically fit” patients with suitable anatomy. Most patients select EVAR because of its early perioperative advantages and the “less invasive” nature of the procedure. At midterm follow-up after 2 and 4 years in the DREAM (Dutch Randomized Endovascular Aneurysm Repair) and EVAR-1 studies, EVAR was associated with a greater number of late complications and secondary reinterventions, and the initial reduction in mortality with EVAR was no longer present within 1 to 2 years.2,5,9 After a mean follow-up of 5 years, EVAR and OSR in the OVER (Open Versus Endovascular Repair) trial had similar survival rates, with EVAR demonstrating improved survival in those younger than 70 years, but not in older patients.6 The development of “endoleaks” (persistent blood flow in the aneurysm sac outside the endograft) is reported in almost 25% of patients at follow-up and is an important cause of aortic rupture after EVAR.2 There are different types of endoleaks (Table 57-1). Type I endoleaks, which result from loss of complete sealing at the proximal (type IA) or distal (type IB) end of the stent-graft, lead to increased pressure in the aneurysm sac and are associated with increased risk for rupture.1 Even though some may seal spontaneously, this problem is ideally corrected during the EVAR procedure. Proximal endoleaks may be treated with extensions, stent placement, or endovascular obliteration of the space, whereas distal endoleaks are treated by extension techniques. Type II endoleaks, the most common, result from retrograde filling of the aneurysm sac by the lumbar or inferior mesenteric arteries. An initial conservative approach is often recommended for type II endoleaks; if sac enlargement is discovered, treatment is recommended. Type III endoleaks are caused by separation of components or disconnection of the endograft and require treatment. Type IV endoleaks are related to blood seeping through porous graft material and are self-limited. Persistence of type I and type II endoleaks may require conversion to open repair, but endovascular approaches may be successful.1 Endotension, an enlarging AAA after EVAR without an endoleak and with a diameter increased to greater than 10 mm, usually requires repair. Late complications of EVAR (endograft migration, limb thrombosis), implant-related complications, and graft infection can also occur. Long-term radiographic surveillance is essential for monitoring the durability of the clinical results. TABLE 57-1 Classification of Endoleaks and Endotension From Moll FL, Powell JT, Fraedrich G, et al: Management of abdominal aortic aneurysms: Clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 41:S1, 2011. Imaging with contrast-enhanced CTA is typically performed at 1 month, 6 months, and annually after implantation of the device.1 A reduced surveillance regimen may be appropriate in cases in which early success is achieved with the newer devices. Additionally, the use of color duplex ultrasonography to detect endoleaks and AAA enlargement may be appropriate for those with stable imaging findings. In conditions in which use of contrast material is prohibited (e.g., renal insufficiency, allergy), duplex ultrasound may be combined with non–contrast-enhanced CT for complete evaluation. Widespread use of EVAR has demonstrated a reduction in early morbidity and mortality in patients with AAAs, especially in older adults. This advantage does not persist in long-term follow-up, however.6 It has been recommended that EVAR be performed at centers with very low in-hospital mortality (<3%) and a primary conversion rate to OSR of less than 2% for elective repair.2 The development of fenestrated and branched endografts is extending EVAR technology to increasingly challenging subsets of patients with aneurysms. TAAs have an estimated incidence of at least 5 to 10 per 100,000 person-years.10 The cause, natural history, and treatment vary depending on the location of the TAA. Aortic root or ascending aortic aneurysms are most common (≈60%), followed by aneurysms of the descending aorta (≈35%) and aortic arch (<10%).10 Thoracoabdominal aortic aneurysm refers to descending thoracic aneurysms that extend distally to involve the abdominal aorta. Causes of TAAs include genetically triggered, degenerative or atherosclerotic, mechanical, inflammatory, and infectious diseases (Table e57-1 Many disorders of the thoracic aorta have an underlying genetic trigger, some of which are associated with widespread syndromic features and others with thoracic aortic disease alone (Table 57-2). These disorders are associated with abnormalities in the aortic media, vascular SMCs, or contractile proteins, and many lead to overactivation of signaling pathways and downstream mediators.12 Such disorders include MFS, LDS, vEDS, familial thoracic aortic aneurysm and dissection syndrome (FTAA/D), bicuspid aortic valve (BAV) disease, Turner syndrome (TS), and the aortopathy associated with many congenital heart diseases. TABLE 57-2 Genetically Triggered Conditions Associated with Aortic Dissection
Diseases of the Aorta
The Normal Aorta
Anatomy and Physiology
Physiology
Evaluation of the Aorta
Aortic Aneurysms
Abdominal Aortic Aneurysms
Pathogenesis
Clinical Features
Diagnostic Imaging
Ultrasound/Computed Tomography/Magnetic Resonance Imaging/Aortography
Screening
Genetics/Molecular Genetics
Natural History
Ruptured Abdominal Aortic Aneurysm
Management
Surveillance/Medical Therapy
Experimental Therapy.
Surgery
Techniques and Outcomes.
Endovascular Abdominal Aortic Aneurysm Repair.
TYPE OF ENDOLEAK
SOURCE OF PERIGRAFT FLOW
I
Attachment site
A
Proximal end of stent-graft
B
Distal end of stent-graft
C
Iliac occluder
II
Branch leaks without attachment site leaks
A
Simple: one patent branch
B
Complex: two or more patent branches
III
Stent-graft defect
A
Junctional leak or modular disconnect
B
Fabric holes
IV
Stent-graft fabric porosity <30 days after placement
Endoleaks (time of detection)
Primary, present from the time of EVAR
Secondary, appearing after previous negative CTA
Endotension
AAA enlargement with increased intrasac pressure after EVAR but without an endoleak visualized on CTA
Thoracic Aortic Aneurysms
Cause and Pathogenesis
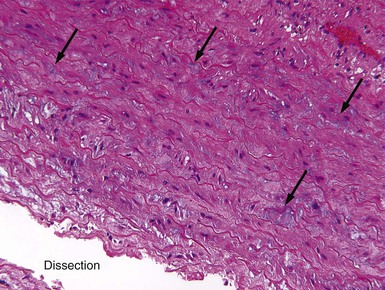
![]() ). Many of the genetic disorders preferentially involve the aortic root and ascending aorta. Smoking, hypertension, age, COPD, coronary disease, and a family history are all risk factors for TAAs.11 Cystic medial degeneration (CMD) describes degeneration and fragmentation of elastic fibers, loss of SMCs, increase in deposition of collagen, and replacement with interstitial “cysts” of mucoid-appearing basophilic-staining extracellular matrix (Fig. e57-1
). Many of the genetic disorders preferentially involve the aortic root and ascending aorta. Smoking, hypertension, age, COPD, coronary disease, and a family history are all risk factors for TAAs.11 Cystic medial degeneration (CMD) describes degeneration and fragmentation of elastic fibers, loss of SMCs, increase in deposition of collagen, and replacement with interstitial “cysts” of mucoid-appearing basophilic-staining extracellular matrix (Fig. e57-1![]() ). CMD of the aorta is present in patients with MFS and many other genetically triggered TAA diseases. In addition, aging is associated with some degree of CMD, a process that may be accelerated by hypertension. These changes lead to progressive weakening of the aortic wall and eventually result in dilation and aneurysm formation.
). CMD of the aorta is present in patients with MFS and many other genetically triggered TAA diseases. In addition, aging is associated with some degree of CMD, a process that may be accelerated by hypertension. These changes lead to progressive weakening of the aortic wall and eventually result in dilation and aneurysm formation.
Genetically Triggered Thoracic Aortic Aneurysm Diseases
Marfan syndrome (MFS)
Autosomal dominant disorder of connective tissue caused by FBN1
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Diseases of the Aorta
57