Disease of Peripheral Vessels
Amjad Al Mahameed
John R. Bartholomew
Introduction
Peripheral arterial disease (PAD) refers to occlusive atherosclerotic disease of the aorta and the lower extremities. It is common in the elderly and among those with risk factors for atherosclerosis, particularly smoking and diabetes mellitus (DM). The classical symptom of PAD is intermittent claudication (IC), defined as pain, aching, cramping, or fatigue in the leg that is triggered by walking, relieved with rest, and reproduced by resuming activity. Patients with PAD may develop ischemic ulcers or rest pain, often referred to as critical limb ischemia (CLI), or an acutely threatened limb, also known as acute limb ischemia (ALI). Recent studies, however, indicate that a significant proportion of individuals with PAD may be asymptomatic or present with atypical leg symptoms that often go unrecognized or are mistaken as part of growing old.
PAD is associated with an increased risk of cardiovascular morbidity and mortality in both symptomatic and asymptomatic individuals. Whereas endovascular interventions and surgical procedures aim at limb salvage or seek to improve functional status and prevent disability, integration of global cardiovascular risk reduction strategies into the care plan is critical to slowing progression, reducing recurrence, and decreasing adverse cardiovascular events and improving survival in this population.
Prevalence
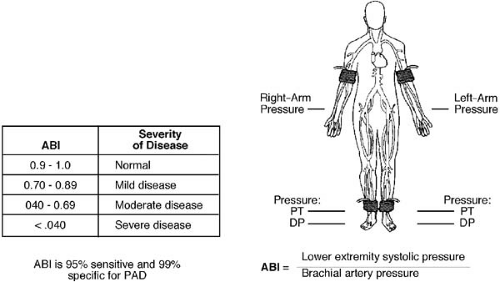
The reported prevalence of PAD varies and is largely dependent on the demographic factors of the population under evaluation and the method of diagnosis. Although earlier accounts based on patients with IC reported prevalence rates of 1.8% to 7% (1,2,3), it is now well recognized that only 10% to 30% of all PAD patients present with this classic symptom (4). Thus, IC is a useful clinical indicator but may underestimate the prevalence of this disease. More recently, the ankle–brachial index (ABI) (the ratio of the systolic blood pressure measured at the ankle arteries to the highest systolic pressure of the brachial arteries) has become the diagnostic test of choice for establishing the presence of PAD (Fig. 108.1). Several recent epidemiologic studies used this method to evaluate the prevalence of PAD in community members of different ages. The National Health and Nutrition Examination Survey (NHANES) enrolled a representative sample of the U.S. population aged 40 years and older and reported an increased prevalence with advancing age (0.9% and 14.5% among individuals aged 40 to 49 years and 70 years and older, respectively) (5). Similarly, an ABI of 0.9 or less was uncommon (2.3% to 4%) in middle-aged (45 to 64 years) individuals enrolled in the Atherosclerosis Risk in Communities Study (ARIC) (6), whereas a relatively higher prevalence (13.4%) was seen in the Cardiovascular Health Study (CHS), which recruited older, Medicare-eligible adults (7). In the PARTNERS program, the prevalence of PAD in 6,979 at-risk individuals (age >70 or 50 to 69 years with a history of smoking or DM) attending primary care practices was also high (29%) (8). Although no difference has been detected in the prevalence of PAD between men and women (1,5,6,7,9) or among whites and Hispanics (8,10), several studies have reported an excess prevalence among non-Hispanic blacks (5,11,12). In the NHANES study, non-Hispanic blacks were at a higher risk for PAD [odds ratio (OR) 2.39] independent of age, gender, smoking status, weight, hypertension (HTN), hypercholesterolemia, DM, and renal function (5).
When both symptomatic and asymptomatic patients are considered, it is estimated that at least 10 million Americans have PAD and 4 million have IC (13). Given the reported annual incidence of IC of approximately 20 per 1,000 in American men and women older than 65 years (14), up to 1.3 million elderly persons are expected to develop disabling IC every 2 years for the next 50 years (15). Consequently, PAD should be recognized as a leading cause of morbidity and an increasing cause of disability in the United States (16). These compelling epidemiologic facts emphasize the importance of awareness, detection, treatment, and prevention of this noncardiac vascular disease (7,8,17,18), and several recent publications have called for better dissemination of this information to the medical community and the public at large
(19,20,21,22).
(19,20,21,22).
Pathophysiology
Atherosclerosis accounts for the vast majority of PAD, whereas uncommon vascular syndromes, such as vasculitis, thromboangiitis obliterans, popliteal entrapment syndrome, and fibromuscular dysplasia, account for less than 10% of cases. Atherosclerotic plaques frequently develop at arterial bifurcations, presumably due to both impaired atheroprotective mechanisms and the effects of disturbed blood flow on endothelial cells (23,24). These plaques are highly cellular and contain intrinsic vascular wall cells (endothelial and smooth muscle cells) and inflammatory cells (monocytes, macrophages, and lymphocytes) in addition to a thrombogenic lipid core that is covered by a fibrous cap (24,25,26). Once the fibrous cap is disrupted, the resultant exposure of the prothrombogenic lipid core can lead to thrombus formation and flow occlusion (24). This and other complex interactions between systemic and local factors at the atherosclerotic lesion site can lead to progression from asymptomatic PAD to IC, CLI, or ALI (24).
The pathophysiology of IC extends beyond an exercise-induced supply–demand mismatch that is caused by hemodynamic abnormalities imposed by arterial luminal stenosis. Metabolic changes also occur in chronically ischemic skeletal muscle groups (Table 108.1) (27,28) and are consistent with an “acquired metabolic myopathy” that manifests clinically as muscle weakness, functional impairment, and walking limitation.
Risk Factors for Peripheral Arterial Disease
Risk factors for PAD are similar to those for atherosclerosis in other vascular beds. Three major factors most strongly associated with PAD are advanced age (>60 years), cigarette smoking, and DM (29). Although the prevalence increases by 1.5- to 2.0 fold for every 10-year increase in age, DM and smoking increase the risk independently by approximately 2- to 4-fold each (9,12,29,30,31). Smoking also results in earlier onset of symptoms (by almost a decade) with an apparent dose–response relationship between the pack/year history and PAD risk (32,33,34,35). For example, in the Framingham Study, the likelihood for PAD increased by 40% for every 10 cigarettes smoked daily, and further analysis revealed that smoking accounts for approximately 75% of all PAD patients (36,37,38). Smokers are also more likely to have poorer survival rates and progress to CLI or amputation compared to nonsmokers (39,40,41,42,43,44). Of significance, the association between smoking and PAD is about twice as strong as that for coronary artery disease (CAD) (35, 36,45).
DM accounts for 45% to 70% of all nontraumatic amputations in the United States (46) and is a stronger risk factor for PAD in women than men (26,47,48). The risk of developing IC in DM in the Framingham cohort increased by 3.5-fold in men and 8.6-fold in women (47), and the 5-year incidence of IC in middle-aged men and women with newly diagnosed DM increased by 2.5- and 5.2-fold, respectively, compared to controls in another study (49). PAD is also more prevalent in individuals with an impaired glucose tolerance test (50), and a significant increase in its risk exists with higher glycosylated hemoglobin (Hgb) A1C levels even among individuals with non–diabetic range dysglycemia (HgbA1C <6%) (51). Finally,
the severity of PAD appears to be related to both the duration of hyperglycemia and glycemic control as demonstrated by a recent meta-analysis (52). Selvin and others proposed that PAD risk increased by 28% to 32% for every 1% increase in HgbA1C (52,53).
the severity of PAD appears to be related to both the duration of hyperglycemia and glycemic control as demonstrated by a recent meta-analysis (52). Selvin and others proposed that PAD risk increased by 28% to 32% for every 1% increase in HgbA1C (52,53).
TABLE 108.1 Metabolic Changes in Chronically Ischemic Skeletal Groups | |
|---|---|
|