Direct Open Revascularization for Renal Artery Occlusive Disease
David B. Wilson
Kimberley J. Hansen
The question of optimal management of atherosclerotic renovascular disease contributing to hypertension or renal insufficiency is unanswerable. There are no prospective, randomized trials comparing available treatment options. In the absence of Level I data, advocates of medical management, percutaneous transluminal renal angioplasty (PTRA), or operative intervention, cite selective clinical data to support their particular views.
A variety of open operative techniques have been used to correct atherosclerotic renovascular disease. From a practical standpoint, three basic operations have been most frequently used: aortorenal bypass, renal artery thromboendarterectomy, and renal artery reimplantation. Although each method may have its proponents, no single approach provides optimal repair for all types of renovascular disease. Aortorenal bypass using saphenous vein is probably the most versatile technique; however, thromboendarterectomy is especially useful for orificial atherosclerosis involving multiple renal arteries. Occasionally, the renal artery will be sufficiently redundant to allow reimplantation; this is probably the simplest technique for renal artery repair.
Operative Exposure
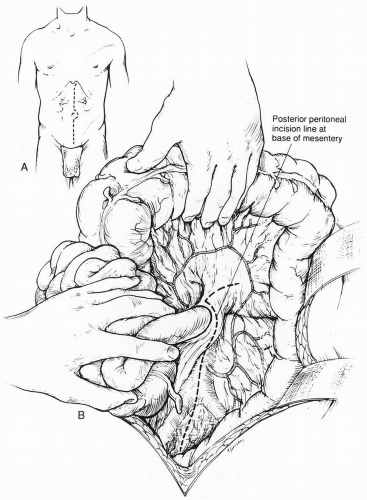
Most frequently, a xiphoid-to-pubis midline abdominal incision is made for operative repair of atherosclerotic renal artery disease. The last 1 or 2 cm proximal incision is made coursing to one side of the xiphoid to obtain full exposure of the upper-abdominal aorta and renal branches. Some type of fixed mechanical retraction is also advantageous, particularly when combined aortorenal procedures are required. Extended flank and subcostal incisions are most commonly reserved for branch renal artery reconstruction following failed endoluminal intervention or for splanchno-renal bypass. When the supraceliac aorta is used as an inflow source, the ipsilateral flank, is elevated and the incision extends from the opposite semilunar line into the flank bisecting the abdominal wall between the costal margin and iliac crest. A left or right visceral mobilization allows access to the renal vasculature and the aortic hiatus. The diaphragmatic crus can be divided, and an extrapleural dissection of the descending thoracic aorta provides access to the T9-10 thoracic aorta for proximal control and anastomosis.
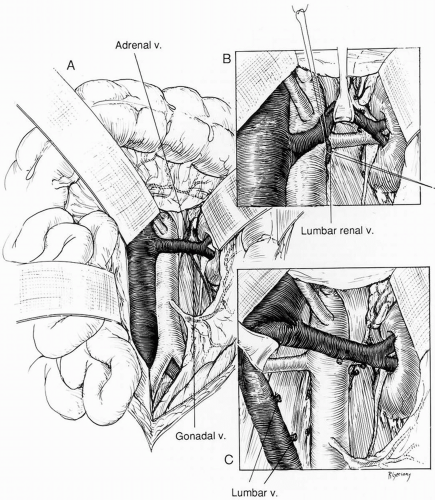
When the midline xiphoid-to-pubis incision is used, the posterior peritoneum overlying the aorta is incised longitudinally and the duodenum is mobilized at the Treitz ligament (Fig. 40-1). During this maneuver, it is important to identify visceral collaterals (i.e., the meandering mesenteric artery) that course at this level. Finally, the duodenum is reflected to the patient’s right to expose the left renal vein. By extending the posterior peritoneal incision to the left along the inferior border of the pancreas, an avascular plane posterior to the pancreas can be entered (Fig. 40-1) to expose the entire left renal hilum. This exposure is of special importance when there are distal renal artery lesions to be managed (Fig. 40-2A). The left renal artery lies posterior to the left renal vein. In some cases, the vein can be retracted cephalad to expose the artery; in other cases, caudal retraction of the vein provides better access. Usually, the gonadal and adrenal veins, which enter the left renal vein, must be ligated and divided to facilitate exposure of the distal artery. Frequently a lumbar vein enters the posterior wall of the left renal vein, and it can be injured easily unless special care is taken during dissection (Fig. 40-2B). The proximal portion of the right renal artery can be exposed through the base of the mesentery by retracting the left renal vein cephalad and the vena cava to the patient’s right (Fig. 40-2C). However, the distal portion of the right renal artery is best exposed by mobilizing the duodenum and right colon medially; the right renal vein is mobilized and usually retracted cephalad in order to expose the artery.
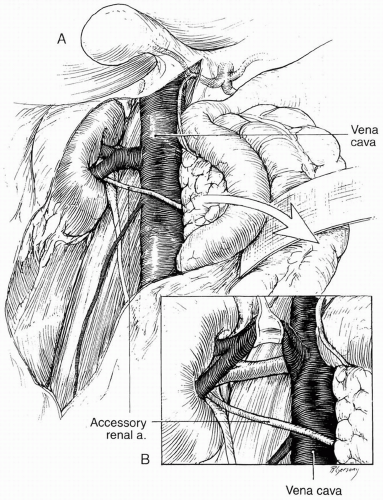
Branch renal artery exposure on the right is achieved by colonic and duodenal mobilization. First, the hepatic flexure is mobilized at the peritoneal reflection. With the right colon retracted medially and inferiorly, a Kocher maneuver mobilizes the duodenum and pancreatic head to expose the inferior vena cava and right renal vein. Typically, the right renal artery is located just inferior to the accompanying vein, which can be retracted superiorly to provide best exposure. Though accessory vessels may arise from the aorta or iliac vessels at any level, all arterial branches coursing anterior to the vena cava should be considered accessory right renal branches and carefully preserved (Fig. 40-3A and 40-3B).
When bilateral renal artery lesions are to be corrected and when correction of a right renal artery lesion or bilateral lesions is combined with aortic reconstruction, these exposure techniques can be modified. Extended aortic exposure may be provided by mobilizing the base of the small bowel
mesentery exposure to allow complete evisceration of the entire small bowel, right colon, and transverse colon. For this extended exposure, the posterior peritoneal incision begins with division of the Treitz ligament and proceeds along the base of the mesentery to the cecum and then along the lateral gutter to the foramen of Winslow (Fig. 40-4A). The inferior border of the pancreas is fully mobilized to enter a retropancreatic plane, thereby exposing the aorta to a point above the superior mesenteric artery. Through this modified exposure, simultaneous bilateral renal endarterectomies, aortorenal grafting, or renal artery attachment to the aortic graft can be performed with complete visualization of the entire aorta and its branches. Another useful technique for suprarenal aortic exposure is partially dividing both diaphragmatic crura as they pass posterior to the renal arteries to their paravertebral attachment. This partial division of the crura allows the aorta above the superior mesenteric artery to be easily visualized and mobilized for suprarenal crossclamping (Fig. 40-4B).
mesentery exposure to allow complete evisceration of the entire small bowel, right colon, and transverse colon. For this extended exposure, the posterior peritoneal incision begins with division of the Treitz ligament and proceeds along the base of the mesentery to the cecum and then along the lateral gutter to the foramen of Winslow (Fig. 40-4A). The inferior border of the pancreas is fully mobilized to enter a retropancreatic plane, thereby exposing the aorta to a point above the superior mesenteric artery. Through this modified exposure, simultaneous bilateral renal endarterectomies, aortorenal grafting, or renal artery attachment to the aortic graft can be performed with complete visualization of the entire aorta and its branches. Another useful technique for suprarenal aortic exposure is partially dividing both diaphragmatic crura as they pass posterior to the renal arteries to their paravertebral attachment. This partial division of the crura allows the aorta above the superior mesenteric artery to be easily visualized and mobilized for suprarenal crossclamping (Fig. 40-4B).
Aortorenal Bypass
Three types of materials are available for aortorenal bypass: autologous saphenous vein, autologous hypogastric artery, and synthetic prosthetic. The decision as to which graft should be used depends on a number of factors. In most instances, the authors use the saphenous vein preferentially. However, if the vein is small (less than 4 mm in diameter) or sclerotic, a synthetic graft may be preferable. A 6 mm, thin-walled polytetrafluoroethylene graft is quite satisfactory when the distal renal artery is of sufficient caliber (≥4 mm). Hypogastric artery autograft is preferred for aortorenal bypass in children when reimplantation is not possible.
Although a distal end-to-side anastomosis may be used, an end-to-end anastomosis between the graft and the distal renal artery provides a better reconstruction in most cases (Fig. 40-5). In bypass procedures, the proximal anastomosis is performed first and the distal renal anastomosis performed secondly to limit renal ischemia. Regardless of the type of distal anastomosis, the proximal aortorenal anastomosis is best performed after excision of an ellipse of aortic wall. This is especially important when the aorta is relatively inflexible due to atherosclerotic involvement. A 5.2 mm aortic punch applied two to three times creates a very satisfactory ellipse in most instances. For both proximal and distal anastomosis, the length of the arteriotomy should be at least three times the diameter of the smaller conduit to avoid late suture-line stenosis.
Thromboendarterectomy
In cases of ostial atherosclerosis of both renal artery origins, simultaneous bilateral endarterectomy may be the most suitable procedure. Endarterectomy may be either transaortic or transrenal. In the latter instance, the aortotomy is made transversely and is carried across the aorta and into the renal artery to a point beyond the visible atheromatous disease. With this method, the distal endarterectomy can be easily assessed and tacked down with mattress sutures under direct vision if necessary. Following completion of the endarterectomy, the arteriotomy is closed. In most patients, this closure is performed with a synthetic patch to ensure that the proximal renal artery is widely patent. For the majority of renal endarterectomies, however, the transaortic technique is used. The transaortic method is particularly applicable in patients with multiple renal arteries that demonstrate ostial disease. In this instance, all visible and palpable renal artery atheroma should end within one centimeter of its aortic origin. Transaortic endarterectomy is performed through a longitudinal aortotomy with sleeve endarterectomy of the aorta and eversion endarterectomy of the renal arteries (Fig. 40-6). When the aortic atheroma is divided flush with adventitia, tacking sutures are not usually required. Alternatively, when combined aortic replacement is planned, the transaortic endarterectomy is performed through the transected aorta. When using the transaortic technique, it is important to mobilize the renal arteries extensively to
allow eversion of the vessel into the aorta. This allows the distal end point to be completed under direct vision.
allow eversion of the vessel into the aorta. This allows the distal end point to be completed under direct vision.
As with arterial thromboendarterectomy at all anatomic sites, the procedure is contraindicated by the presence of preaneurysmal degeneration of the aorta and the presence of transmural calcification. The latter condition can be subtle and missed unless careful attention is given to gentle palpation of the aorta and renal arteries. Atheroma complicated by transmural calcification resembles fine-grade sandpaper on palpation. Endarterectomy in this setting is characterized by numerous sites of punctate bleeding after blood flow is restored.
Renal Artery Re-implantation
After the renal artery has been dissected from the surrounding retroperitoneal tissue the vessel may be somewhat redundant. When the renal artery stenosis is orificial and there is sufficient vessel length, the renal artery can be transected and re-implanted into the aorta at a slightly lower level. The renal artery must be spatulated and a portion of the aortic wall removed as in renal artery bypass. When performed during combined aortic replacement in adults, the renal artery to graft anastomosis is usually performed first after the proximal aortic anastomosis, followed by distal aortic reconstruction.
Splanchno-renal Bypass
Splanchno-renal bypass and other indirect revascularization procedures have received increased attention as an alternative method for renal revascularization. The authors do not believe that these procedures demonstrate durability equivalent to direct aortorenal reconstructions, but they are useful in a highly select subgroup of high-risk patients.
Hepatorenal Bypass
A right subcostal incision is used to perform hepatorenal bypass. The lesser omentum is incised to expose the hepatic artery both proximal and distal to the gastroduodenal artery. Next, the descending duodenum is mobilized by the Kocher maneuver, the inferior vena cava is identified, the right renal vein is identified, and the right renal artery is exposed either immediately cephalad or caudad to the renal vein.
A greater saphenous vein graft is usually used to construct the bypass. The hepatic artery anastomosis of the vein graft can be placed at the site of the amputated stump of the gastroduodenal artery; however, this vessel may serve as an important collateral for intestinal perfusion. Therefore, the proximal anastomosis is usually made to the common hepatic artery. After completion of this anastomosis, the renal artery is transected and brought anterior to the vena cava for end-to-end anastomosis to the graft.
Splenorenal Bypass
Splenorenal bypass can be performed through a midline or a left subcostal incision. The posterior pancreas is mobilized by reflecting the inferior border cephalad. A retropancreatic plane is developed and the splenic artery mobilized from the left gastroepiploic artery to the level of its branches. The left renal artery is exposed cephalad to the left renal vein after division of the adrenal branch. After the splenic artery has been mobilized, it is divided distally, spatulated, and anastomosed end-to-end to the transected renal artery. Alternatively, a saphenous vein graft may be used as a bypass from the splenic artery.
Ex Vivo Reconstruction
In part, operative strategy for renal artery repair is determined by the exposure required and the anticipated period of renal ischemia. When reconstruction can be accomplished with less than 40 minutes ischemia, an in situ repair is undertaken without special measures for renal preservation. When longer periods of ischemia are anticipated, one of two techniques for hypothermic preservation of the kidney are considered; these include renal mobilization without renal vein transection or ex vivo repair and orthotopic replacement in the renal fossa.
Ex vivo management is necessary when extensive exposure is required for prolonged
periods. For management of atherosclerotic renovascular disease, ex vivo repair is usually reserved for branch renal artery repair after failed endovascular intervention or associated branch renal artery aneurysms. Several methods of ex vivo hypothermic perfusion and reconstruction are available. A midline xiphoid-to-pubis incision is used for most renovascular procedures and is preferred when autotransplantation of the reconstructed kidney or combined aortic reconstructions are to be performed. When isolated branch renal repair with orthotopic replacement is planned, an extended flank incision is made parallel to the lower rib margin and carried to the posterior axillary line as described earlier. The ureter is always mobilized to the pelvic brim. An elastic sling is placed around the ureter to prevent perfusion from ureteric collaterals and subsequent renal rewarming.
periods. For management of atherosclerotic renovascular disease, ex vivo repair is usually reserved for branch renal artery repair after failed endovascular intervention or associated branch renal artery aneurysms. Several methods of ex vivo hypothermic perfusion and reconstruction are available. A midline xiphoid-to-pubis incision is used for most renovascular procedures and is preferred when autotransplantation of the reconstructed kidney or combined aortic reconstructions are to be performed. When isolated branch renal repair with orthotopic replacement is planned, an extended flank incision is made parallel to the lower rib margin and carried to the posterior axillary line as described earlier. The ureter is always mobilized to the pelvic brim. An elastic sling is placed around the ureter to prevent perfusion from ureteric collaterals and subsequent renal rewarming.
Table 40-1 Solution for Cold Perfusion Preservation of the Kidney | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Gerota fascia is opened with a cruciate incision, and the kidney is completely mobilized and the renal vessels divided (Fig. 40-7). The kidney is placed in a plastic sling, packed in ice slush, and perfused with a chilled renal preservation solution. Continuous perfusion during the period of total renal ischemia is possible with perfusion pump systems, and it may be superior for prolonged renal preservation. However, simple intermittent flushing with a chilled preservation solution provides equal protection during the shorter periods (2 to 3 hours) required for ex vivo dissection and branch renal artery reconstructions. For this technique, we refrigerate the preservative overnight, add additional components (Table 40-1) immediately before use to make up a liter of solution, and hang the chilled (5 to 10° C) solution at a height of at least 2 meters. Three to five hundred milliliters of solution are flushed through the kidney immediately after its removal from the renal fossa until the venous effluent is clear. As each anastomosis is completed, the kidney is perfused with an additional 150 to 200 mL of solution. In addition to maintaining satisfactory hypothermia, periodic perfusion demonstrates suture line leaks that are repaired prior to re-implantation. With this technique, renal core temperatures are maintained at 10° C or below throughout the period of reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree