Direct, Open Revascularization for Aortoiliac Occlusive Disease
David C. Brewster
The infrarenal abdominal aortic and iliac arteries are among the most common sites of the occlusive atherosclerotic disease that is responsible for symptomatic arterial insufficiency of the lower extremities. Because atherosclerosis is a systemic process, patients with aortoiliac disease frequently have coexistent disease below the inguinal ligament. Nonetheless, the disease is usually segmental in distribution and therefore amenable to effective surgical treatment. Even in patients with significant concomitant infrainguinal disease, successful revascularization of the aortoiliac segment frequently leads to adequate improvement of ischemic symptoms.
Since the introduction of the initial methods of aortoiliac reconstruction more than 40 years ago, improvements in surgical techniques, graft materials, and perioperative care have all contributed to significant reduction of peri-operative morbidity/mortality and excellent long-term results in terms of both graft patency and symptom relief. Such results have clearly established aortobifemoral bypass as the procedure of choice for the majority of patients with aortoiliac occlusive disease.
Indications
The most common indications for aortoiliac revascularization are severe intermittent claudication and limb-threatening ischemia secondary to atherosclerotic occlusive disease involving the infrarenal aorta and both iliac systems. Almost all patients with severe ischemia manifested by rest pain or tissue loss are found to have multilevel disease involving the aortoiliac and infrainguinal arterial segments. In such patients, initial correction of inflow disease by aortoiliac revascularization is appropriate. Establishing adequate flow to the profunda femoris affords satisfactory clinical relief of the ischemic symptoms in 75% to 80% of patients despite the uncorrected infrainguinal disease.
A less frequent but well-recognized indication is peripheral atheromatous embolization (blue toe syndrome) from proximal ulcerated atherosclerotic plaques in the aortoiliac system. If a likely source of such events can be identified by arteriographic evaluation, aortobifemoral bypass with exclusion of the native aortoiliac segment is often advisable, even if the lesions are not hemodynamically significant.
Pre-operative Assessment
There are numerous options for revascularization in patients with aortoiliac disease. Selection of the most appropriate method depends largely on two factors: (a) the patient’s surgical risk and (b) the extent and distribution of occlusive disease. Aortobifemoral bypass provides superior long-term results in terms of durability and sustained symptom relief. However, it is a major operative procedure that may not be well suited for patients with serious comorbid medical conditions. Thus, careful pre-operative evaluation is important. For patients with relatively limited areas of disease, particularly for unilateral iliac disease, alternative “lesser” procedures, such as percutaneous transluminal angioplasty, femorofemoral bypass, or unilateral iliofemoral grafting, may be more appropriate. For high-risk patients with bilateral iliac disease or patients with relative contraindications to direct aortic reconstruction, such as heavy retroperitoneal scarring or contamination, extra-anatomic axillobifemoral bypass may be a better alternative. However, the long-term patency rates of these grafts are inferior.
Careful pre-operative evaluation is important to identify and potentially correct any comorbid conditions that might increase the risk of revascularization. Although somewhat controversial, pre-operative evaluation of coronary artery disease (CAD) is important for patients with evidence of ischemic heart disease by either history or electrocardiogram. If the noninvasive screening studies such as exercise stress testing or adenosine thallium suggest significant myocardial ischemia, pre-operative coronary arteriography may be advisable. Similarly, significant abnormalities of pulmonary, renal, or coagulation function should be routinely evaluated and optimized.
High-quality pre-operative arteriography remains of paramount importance before aortoiliac revascularization. In addition to standard anteroposterior views, lateral and oblique images of the visceral, iliac, and profunda femoral vessels should be obtained. Complete, bilateral infrainguinal arteriograms are also generally advisable, both for operative planning and minimizing the chances of technical misadventure. In patients felt to be at high risk for conventional, contrast arteriography, magnetic resonance arteriography may serve as an alternative.
Noninvasive vascular studies are generally performed in all patients. Segmental lower extremity pressure measurements and pulse volume recordings (plethysmography) confirm the diagnosis, quantify its severity, and establish a baseline for assessing the results of revascularization. Furthermore, exercise stress testing can serve
to quantify the walking distances among claudicants.
to quantify the walking distances among claudicants.
Operative Procedure
Aortobifemoral Bypass
Two to four units of packed red blood cells are typed and cross-matched. Pre-operative donation of two units of autologous blood is encouraged and is generally possible in elective circumstances. Adequate pre-operative hydration is ensured, including administration of intravenous fluids if clinically necessary. A broad-spectrum prophylactic antibiotic such as cefazolin (1 g) is given intravenously 1 to 2 hours before surgery and continued for 1 or 2 days postoperatively. In patients with infected lower-extremity ischemic lesions or any other possible source of bacteremia, culture-specific oral antibiotics are often started several days before the operation.
The patient is placed supine on the operating table with both arms extended at right angles on armboards to permit appropriate monitoring during anesthesia and to establish vascular access. A radial artery cannula is inserted for continuous blood pressure monitoring and arterial blood gas determinations. A Swan-Ganz catheter is inserted in selective patients based upon the pre-operative assessment of cardiac and renal function. Most patients undergoing aortic surgery in contemporary practice are anesthetized with a combination of epidural narcotics and inhalation agents (combined general and epidural technique). Continuation of the epidural analgesia in the early postoperative period for pain control has had a significant impact on limiting the systemic narcotic use and has reduced the associated complications after aortic surgery. Warmed intravenous fluids and any one of the commercially available external wrapping devices (such as the Bair Hugger) can be very useful in maintaining body temperature and avoiding the potentially deleterious effects of hypothermia secondary to heat loss.
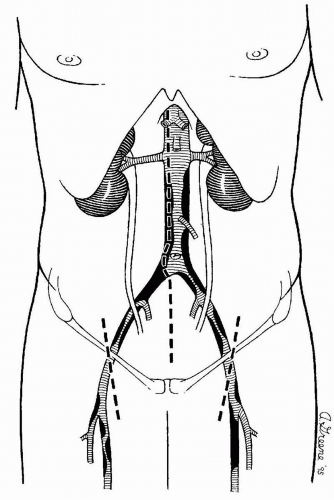
The infrarenal abdominal aorta may be exposed for the aortofemoral reconstruction using a variety of incisions. A long vertical midline incision is used most often (Fig. 44-1) and is generally preferred because it is fast, easy to close, and affords maximal exposure and thereby technical flexibility for most patients. Alternatively, a retroperitoneal approach may be employed, and it may be advantageous in obese patients and those with hostile abdomens or previous aortic procedures.
I generally prefer to expose the femoral vessels before making the abdominal incision to minimize the length of time that the abdomen is open, thereby limiting evaporative fluid and heat losses. As shown in Figure 44-1, the groin incisions are oriented slightly obliquely and placed so that the cephalad third of the incision lies above the inguinal ligament. Retraction by an assistant during construction of the femoral anastomosis is generally unnecessary when the incisions are placed in this location.
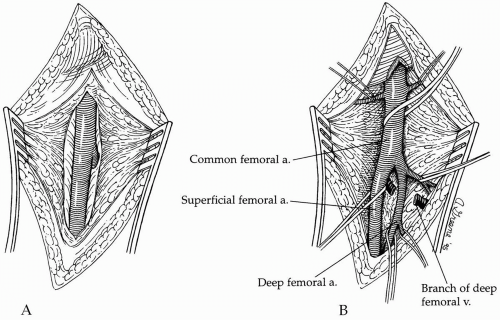
Dissection is carried directly onto the anterior surface of the common femoral artery and then cephalad to the inguinal ligament. Lymph nodes and/or lymphatic tissue are best divided between clamps and then suture-ligated to minimize the possibility of a postoperative lymphatic leak with its associated risk of wound or graft infection. The caudal border of the inguinal ligament is partially divided directly over the femoral artery to ensure ample space for tunneling of the graft limb without compression. Dissection is then carried caudally to expose the common femoral artery bifurcation, and the proximal aspect of both the superficial and profunda femoral arteries are encircled with vessel loops (Fig. 44-2). Similarly, any sizable side branches of the femoral arteries are preserved and controlled with such loops. If significant occlusive disease is found in the proximal profunda femoris artery on the pre-operative arteriogram or by intra-operative palpation, the vessel is exposed further caudally beyond the significant disease to allow concomitant profundaplasty at the time of distal anastomosis. This usually requires exposing an additional 2 to 3 cm of the vessel and necessitates division of one or more branches of the profunda femoral vein that typically cross the anterior surface of the proximal artery.
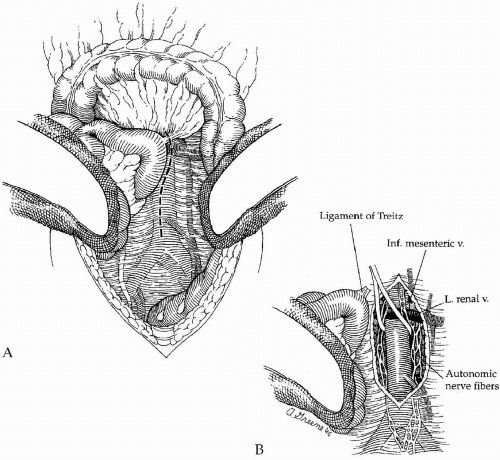
A midline abdominal incision is then created extending from the xiphoid to the pubis. After careful exploration of the intra-abdominal organs, the transverse colon and greater omentum are elevated and retracted cephalad, and the entire small bowel is eviscerated and displaced to the right (Fig. 44-3). The descending and sigmoid portions of the colon are retracted laterally and caudally. After these maneuvers, the posterior parietal peritoneum overlying the infrarenal
aorta is visualized, and this is incised along the longitudinal axis of the aorta starting between the duodenum on the patient’s right and the inferior mesenteric vein to the left (Fig. 44-3A). Care is taken to avoid the plexi of autonomic nerve fibers (Fig. 44-3B) that course primarily along the left anterolateral aspect of the infrarenal aorta and the proximal left common iliac artery. Careful dissection helps preserve these autonomic nerves and helps reduce the incidence of postoperative sexual dysfunction in male patients.
aorta is visualized, and this is incised along the longitudinal axis of the aorta starting between the duodenum on the patient’s right and the inferior mesenteric vein to the left (Fig. 44-3A). Care is taken to avoid the plexi of autonomic nerve fibers (Fig. 44-3B) that course primarily along the left anterolateral aspect of the infrarenal aorta and the proximal left common iliac artery. Careful dissection helps preserve these autonomic nerves and helps reduce the incidence of postoperative sexual dysfunction in male patients.
The retroperitoneal incision is extended cephalad and the ligament of Treitz is divided. This allows mobilization of the fourth portion of the duodenum off the aorta and visualization of the left renal vein as it crosses anterior to the aorta just below the renal artery origins. The left renal vein is an important landmark because the proximal graft anastomosis should be placed as close to it (and the renal arteries) as possible. This serves to minimize the potential for recurrent occlusive disease in the infrarenal aorta above the proximal anastomosis that could potentially compromise the patency of the graft. The aortic dissection is extended distally just beyond the origin of the inferior mesenteric artery. This extent of aortic exposure is sufficient to allow both construction of a proper proximal graft anastomosis and tunneling of each graft limb to the groin. Furthermore, it minimizes the dissection in the region of the aortic bifurcation itself, thereby reducing the possibility of autonomic nerve injury as described.
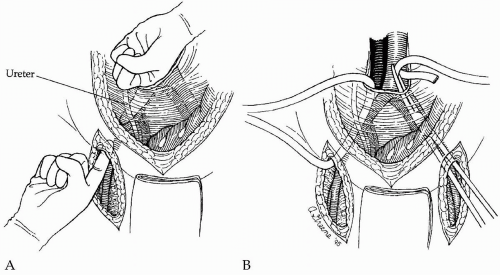
After completion of the aortic and femoral dissections, retroperitoneal tunnels are next made for passage of each graft limb from the aorta to the groins. Such tunnels are best made by gentle blunt dissection using both index fingers simultaneously, with one extending from the groin cephalad and the other from the aortic bifurcation caudal (Fig. 44-4A). Dissection should be kept on a plane directly anterior to the common and external iliac vessels to guarantee that the graft is subsequently placed posterior to the ureter. This is important because passage of the graft anterior to the ureter may lead to compression and obstruction of the ureter with hydronephrosis. When starting the tunnel in the groin, care must be taken not to tear the circumflex iliac venous branches that cross the distal external iliac artery just above the inguinal ligament. After appropriate tunnels have been created to both groins, a long blunt-tipped clamp is placed through the tunnel and a Penrose drain drawn through the tunnel (Fig. 44-4B). Elevation of both ends of the drain facilitates later passage of the graft limbs.
A variety of prosthetic grafts are available for the aortobifemoral bypass, including conventional Dacron (knitted and woven), coated Dacron (collagen, albumin, or gelatin), and polytetrafluoroethylene (PTFE). Available data do not suggest that any graft material or construction has superior patency, and selection is based mostly on the surgeon’s personal preference.
Use of a properly sized graft is important to minimize the possibility of sluggish flow and deposition of excessive laminar thrombus that is likely to occur in an oversized graft. For aortoiliac occlusive disease, a 16 × 8 mm bifurcated graft (body diameter of 16 mm and limb diameter of 8 mm) is used most commonly, but a 14 × 7 mm prosthesis may be more suitable for patients with a relatively small-caliber aortoiliac segment (predominantly women). For most patients, an end-to-end aortic anastomosis (Fig. 44-5) is preferred for several reasons. First, because all blood flows through the graft, there is less chance of “competitive” flow through the native aortoiliac vessels that may potentially increase the incidence of graft limb thrombosis. Second, an end-to-end anastomosis is theoretically hemodynamically superior. It is associated with less
peri-anastomotic turbulence and therefore a smaller likelihood of developing recurrent atheroma or an anastomotic aneurysm. In addition, the end-to-end anastomosis is less likely to cause distal atheromatous embolization and is easier to cover with retroperitoneal tissue after implantation than the end-to-side anastomosis that tends to protrude anteriorly off the aorta. This consideration may reduce the potential for late graft-enteric fistula formation. However, end-to-side anastomosis may be advantageous in certain anatomic patterns of disease, as described below.
peri-anastomotic turbulence and therefore a smaller likelihood of developing recurrent atheroma or an anastomotic aneurysm. In addition, the end-to-end anastomosis is less likely to cause distal atheromatous embolization and is easier to cover with retroperitoneal tissue after implantation than the end-to-side anastomosis that tends to protrude anteriorly off the aorta. This consideration may reduce the potential for late graft-enteric fistula formation. However, end-to-side anastomosis may be advantageous in certain anatomic patterns of disease, as described below.
After intravenous administration of 5,000 to 7,500 units of heparin, appropriate vascular clamps are applied to the aorta just caudal to the left renal vein and immediately caudal or cephalad to the inferior mesenteric artery (Fig. 44-5A). The aorta is then transected, and a 3- to 4-cm-long segment between the clamps is resected. Any patent lumbar artery branches arising from this segment are clamped and ligated. Care should be taken to maintain a resection plane immediately on the posterior wall of the aorta to prevent injury and troublesome bleeding from the adjacent lumbar veins.
The transected distal aortic end is next oversewn in two layers with a 3-0 vascular suture (Fig. 44-5B). If this segment is heavily calcified or diseased, a limited endarterectomy of the calcific plaque and Teflon-pledgeted sutures may be necessary to achieve a secure and hemostatic closure. The body of the bifurcated graft is tailored, leaving approximately 3 to 4 cm from the bifurcation. This allows the short graft body to be situated in the bed of the resected aortic segment and facilitates closure of the retroperitoneum over the graft and separation of the anastomosis from the duodenum and other viscera. The short graft body also serves to advance the level of the graft bifurcation more cephalad and diminishes the takeoff angle of the graft limbs, thereby reducing the chance of kinking the graft at the origin of the limbs.
The divided proximal end of the aorta is inspected and thrombus or loose atheromatous debris removed. Standard graft anastomosis
using a running 3-0 monofilament vascular suture is then performed (Fig. 44-5D). I usually start the anastomosis in the midline posteriorly using a double-armed suture. The anastomosis is performed in a running fashion extending both clockwise and counterclockwise approximately half the circumference of the aorta. A similar suture is then started on the midanterior aspect of the anastomosis and is similarly run in opposite directions. The anterior and posterior sutures are then tied to each other on the lateral aspects of the aorta to complete the anastomosis.
using a running 3-0 monofilament vascular suture is then performed (Fig. 44-5D). I usually start the anastomosis in the midline posteriorly using a double-armed suture. The anastomosis is performed in a running fashion extending both clockwise and counterclockwise approximately half the circumference of the aorta. A similar suture is then started on the midanterior aspect of the anastomosis and is similarly run in opposite directions. The anterior and posterior sutures are then tied to each other on the lateral aspects of the aorta to complete the anastomosis.
If the proximal, infrarenal aorta is significantly diseased and its lumen compromised, I often perform a thromboendarterectomy of the aortic stump up to the level of the proximal clamp (Fig. 44-5C). The remaining adventitial layer is often quite thin, but it usually holds sutures well and allows a technically perfect anastomosis. In this circumstance, I prefer to use an interrupted mattress suture technique with each suture bolstered by a Teflon pledget (Fig. 44-6).
After completion of the aortic anastomosis, the graft is clamped with an atraumatic vascular clamp (Fogarty soft-jawed clamp), and the proximal anastomosis is tested by slow release of the proximal aortic clamp. If inspection reveals any leaks or defects, they are repaired with interrupted mattress sutures with pledgets. After a hemostatic and secure anastomosis has been verified, the proximal aortic clamp is reapplied and the graft thoroughly suctioned to remove any clot or debris.
Attention is then directed to the femoral region. The Penrose drains, previously placed in the graft tunnels, are elevated, and a long blunt-tipped, slightly curved clamp, such as a large DeBakey aortic clamp, is passed from each groin incision to the region of the aortic dissection. The distal end of each femoral graft limb is then grasped with the clamp under direct vision, and each graft limb is pulled down through the tunnel (Fig. 44-7A




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree