, Aaron L. Baggish2 and Aaron L. Baggish3
(1)
Harvard Medical School Cardiovascular Performance Program, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Cardiovascular Performance Program, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Cardiomyopathies represent a heterogeneous group of heart muscle diseases that are a major cause of morbidity and mortality. Classification schemes for cardiomyopathy have been complex, and efforts have been made to classify the disease states based on myocardial characteristics and etiologies. The etiology, diagnosis, and management of dilated cardiomyopathy (DCM), restrictive/infiltrative cardiomyopathy, and hypertrophic cardiomyopathy (HCM) are the subject of this chapter and several features of each form of cardiomyopathy are highlighted in Table 17-1.
Electronic supplementary material
The online version of this chapter (doi:10.1007/978-1-4471-4483-0_17) contains supplementary material, which is available to authorized users.
Abbreviations
ACE
Angiotensin converting enzyme
AF
Atrial fibrillation
ANA
Anti-nuclear antibody
AR
Aortic regurgitation
ARB
Angiotensin receptor blocker
ARVC
Arrhythmogenic right ventricular cardiomyopathy
BNP
B-type natriuretic peptide
CAD
Coronary artery disease
CMV
Cytomegalovirus
CRT
Cardiac resynchronization therapy
CT
Computed tomography
CXR
Chest x-ray
DCM
Dilated cardiomyopathy
E’
Early peak diastolic tissue velocity
ECG
Electrocardiogram
HCM
Hypertrophic cardiomyopathy
HIV
Human immunodeficiency virus
HOCM
Hypertrophic obstructive cardiomyopathy
ICD
Implantable cardioverter defibrillator
LV
Left ventricle or left ventricular
LVEF
Left ventricular ejection fraction
LVH
Left ventricular hypertrophy
LVOT
Left ventricular outflow tract
MR
Mitral regurgitation
MRI
Magnetic resonance imaging
NTproBNP
N-terminal pro B-type natriuretic peptide
RV
Right ventricle or right ventricular
SAM
Systolic anterior motion
SCD
Sudden cardiac death
SPEP
Serum protein electrophoresis
Introduction
Cardiomyopathies represent a heterogeneous group of heart muscle diseases that are a major cause of morbidity and mortality [1, 2]. Classification schemes for cardiomyopathy have been complex, and efforts have been made to classify the disease states based on myocardial characteristics and etiologies [3]. The etiology, diagnosis, and management of dilated cardiomyopathy (DCM), restrictive/infiltrative cardiomyopathy, and hypertrophic cardiomyopathy (HCM) are the subject of this chapter and several features of each form of cardiomyopathy are highlighted in Table 17-1.
Table 17-1
Typical features of the various forms of cardiomyopathy
Parameter | DCM | Restrictive/infiltrative | HCM |
|---|---|---|---|
Definition | Ventricular dilation/ impaired contractility | Impaired ventricular filling due to decreased compliance | Marked LVH in the absence of a pressure load |
Common causes | CAD | Myocardial infiltration (amyloid, sarcoid, hemochromatosis) | Familial |
Valve disease | Endomycardial (Löeffler’s endocarditis) | Sporadic | |
Idiopathic | |||
Familial | |||
Infectious | |||
Toxin | |||
Classic echocardiographic findings | Ventricular dilation ± thrombus | ʿ wall thickness | LVH (asymmetric) |
¯ LVEF | Biatrial enlargement | SAM | |
MR | LVOT gradient |
DCM
Definition
Ventricular dilation and impaired contractility (left ventricle [LV] and/or right ventricle [RV]); typically normal LV wall thickness
Prevalence 1:2,500
Etiology [4]
Cardiac causes: Ischemia/coronary artery disease (CAD); valvular heart disease (i.e. chronic volume overload from aortic regurgitation [AR] or mitral regurgitation [MR])
Idiopathic (possibly undiagnosed genetic mutations [titin] or infectious causes)
Familial (20–35 % of DCM): mutations in contractile sarcomeric, nuclear envelop, and transcriptional coactivator proteins
Defined as DCM of unknown cause in two or more closely related family members
Infectious
Viral (i.e. Coxsackie, Adenovirus, cytomegalovirus [CMV], human immunodeficiency virus [HIV])
Bacterial (i.e. Lyme), Fungal, Parasitic (Chagas disease, typically LV apical aneurysm)
Toxic
Alcohol, cocaine
Chemotherapeutic agents: anthracyclines (increased risk with dose >550 mg/m2), cyclophosphamide, trastuzumab
Tachycardia-mediated: proportional to heart rate and duration of tachycardia
Stress-induced (Takotsubo): classically apical ballooning (other variants possible); post menopausal women in response to psychological or physiological stressor
LV noncompaction: prominent trabeculations, particularly in LV apex
Infiltrative cardiomyopathy: can present as a mix of DCM and restrictive cardiomyopathy; LV systolic dysfunction more common in late-stage disease.
Arrhythmogenic right ventricular cardiomyopathy (ARVC): fibrofatty tissue replacement, can also involve the LV
Metabolic: hypothyroidism, pheochromocytoma, acromegaly, thiamine deficiency
Peripartum: final month of pregnancy to first 5 months after delivery
Autoimmune
Collagen vascular disease (i.e. systemic lupus erythematosus, scleroderma, polymyositis, rheumatoid arthritis, polyarteritis nodosa)
Idiopathic giant cell myocarditis: can be fulminant in presentation
Eosinophilic: hypersensitivity (mild) or acute necrotizing (severe)
History/Physical Examination and Diagnostic Evaluation
Chest pain with certain etiologies (coronary artery disease, myocarditis)
Elicit history of alcohol or drug use, current or past exposure to chemotherapy, and the ability of the patient to perform daily activities.
Careful family history for ≥3 generations
Symptoms and signs of left and/or right sided heart failure (dyspnea, orthopnea, jugular venous distention, lower extremity edema)
Diffuse and laterally displaced point of maximal impulse, S3 gallop, murmur (i.e. MR)
Initial diagnostic evaluation
12-lead electrocardiogram (ECG): Evaluate for poor R wave progression, Q waves, left atrial enlargement, bundle branch block, atrial fibrillation (AF)
Chest x-ray (CXR): Increased cardiac silhouette, pleural effusions, Kerley B lines
Transthoracic echocardiogram (Fig. 17-1 and Video 17-1): LV dilation, decreased LV ejection fraction (LVEF), global or regional LV hypokinesis, MR (papillary muscle displacement and incomplete mitral valve closure), RV dilation and hypokinesis, LV thrombus
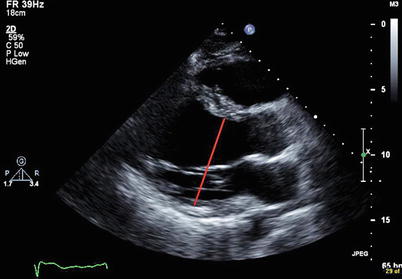
Figure 17-1
Transthoracic echocardiogram from a patient with DCM (parasternal long-axis view) demonstrating LV dilation (red line shows the increased LV inner dimension at end-diastole)
Laboratory studies: complete blood count, serum electrolytes, blood urea nitrogen and serum creatinine, fasting blood glucose or hemoglobin A1C, urinalysis, lipid profile, liver function tests, and thyroid-stimulating hormone.
Measurement of natriuretic peptides (BNP and NT-proBNP) can be useful in the urgent care setting in patients in whom the diagnosis of heart failure is uncertain.
Disease-specific evaluation
Ischemic (CAD):
Stress test: useful if negative, but can have high false positive rate, even with imaging
Coronary computed tomography (CT) angiogram: most useful when low pre-test probability
Coronary angiography [5]
Should be performed in patients with angina or ischemia unless the patient is not eligible for revascularization of any kind (Class I, Level of Evidence B)
Reasonable for patients who have chest pain that may or may not be cardiac in origin who have not had evaluation of their coronary anatomy and who have no contraindication to revascularization (Class IIa, Level of Evidence C)
Reasonable in patients who have known or suspected CAD but who do not have angina, unless the patient is not eligible for revascularization of any kind (Class IIa, Level of Evidence C)
Cardiac magnetic resonance imaging (MRI): useful in evaluation of myocarditis or infiltrative disease
Iron studies, anti-nuclear antibody (ANA), serum protein electrophoresis (SPEP), HIV, selenium, thiamine, etc. based on clinical suspicion for specific causes
Endomyocardial biopsy [6]
New-onset heart failure of <2 weeks’ duration with hemodynamic compromise (Class I, Level of Evidence B)
New-onset heart failure of 2 weeks’ to 3 months’ duration and new ventricular arrhythmias, second- or third-degree heart block, or failure to respond to usual care within 1–2 weeks (Class I, Level of Evidence B)
Should not be performed as a part of routine evaluation (Class III, Level of Evidence C) [5]
Treatment and Prognosis
Identification and treatment of underlying cause if possible
See Chaps. 14 and 15 for detailed treatment including medical therapy (β-blocker, angiotensin converting enzyme [ACE] inhibitor or angiotensin receptor blocker [ARB], aldosterone antagonist), device therapy (implantable cardioverter defibrillator [ICD], cardiac resynchronization therapy [CRT])
Consideration of reversibility is needed before implantation of device therapy
Immunosuppression for giant cell myocarditis, eosinophilic disease, collagen vascular disease and peripartum cardiomyopathy
Prognosis depends on etiology, worst for ischemic cardiomyopathy [4]; overall, DCM most frequent cause of heart transplantation
Screening of family members for familial DCM (after other more common causes, i.e. CAD, cardiotoxic agents, etc.) have been excluded [7]
Genetic testing should be considered for the 1 most clearly affected person in a family to facilitate family screening and management
Clinical screening (history, physical exam, ECG, echocardiogram) for DCM in asymptomatic 1st degree relatives is recommended; interval of screening depends on genotype status
Genetic and family counseling is recommended for all patients and families with familial DCM
Restrictive and Infiltrative Cardiomyopathy
Definition
Impaired ventricular filling (restrictive filling) due to decreased compliance in the absence of pericardial disease
Normal or decreased volume of both ventricles associated with biatrial enlargement; normal or increased LV wall thickness
Etiology [8]
Myocardial
Infiltrative
Amyloidosis [9]: primary (AL), familial (transthyretin), senile; more common in males, average age of presentation approximately 60 years
evaluate for systemic signs and symptoms (nephrotic syndrome, peripheral neuropathy, macroglossia, etc.).
Sarcoidosis: conduction abnormalities, arrhythmia (i.e. ventricular tachycardia); clinical evidence of myocardial involvement in ∼5 % of patients with sarcoidosis (20–30 % show cardiac involvement at autopsy) [10]< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


