We tested whether the “Lund” (LU) electrode-placement system compared to the Mason-Likar (M-L) electrode-placement system would produce waveforms more similar to those of standard electrocardiograms (ECGs) with regard to the QRS axis in the frontal plane and QRS changes of inferior myocardial infarction (MI). We also tested whether LU was more noise immune than standard, and whether the noise immunities of the LU and M-L systems were comparable. Four 12-lead ECGs were recorded in 80 patients—2 standard ECGs, 1 LU ECG, and 1 M-L ECG. Further, 6 ECGs were recorded for 11 patients and 9 healthy volunteers—2 standard, 2 LU, and 2 M-L ECGs—while the subjects performed limb movements. Three electrocardiographic readers made blinded assessments of noise levels. QRS scores in patients with inferior MI differed significantly between standard and M-L ECGs but not between standard and LU ECGs. Few of those without QRS changes of MI received QRS scores, but not more often on LU ECGs than on standard ECGs, and never on M-L ECGs. QRS axis differences were small between standard and LU ECGs, but large between standard and M-L ECGs. The LU system was significantly more noise immune than the standard, whereas the difference in noise immunity between the M-L and LU systems was not significant. In conclusion, the results indicate that LU might constitute a uniform convention for “diagnostic” ECGs and for monitoring electrocardiographic applications.
In clinical electrocardiography standardization of electrode positions is insufficient. Because criteria used for electrocardiographic interpretation are based on distal limb electrode placement, this can lead to misinterpretations with the risk of potentially serious consequences for the patient. A uniform convention for electrocardiographic recording would be worth striving for. The primary aim of the present study was to test the hypothesis that, compared to the Mason-Likar (M-L) electrode-placement system, the “Lund” (LU) electrode-placement system would produce electrocardiographic waveforms with a closer morphologic relation to waveforms obtained from the standard system, with regard to QRS axis in the frontal plane and QRS-estimated inferior myocardial infarct (MI) size, for patients with (1) “normal” electrocardiogram (ECG), (2) left QRS axis deviation, or (3) QRS changes of inferior MI on the standard ECG. The secondary aim was to test the hypothesis that the LU electrode-placement system is more noise immune than the standard, and that the noise immunities of the LU and M-L electrode-placement systems are comparable. If the 2 aims were attained, the LU system could qualify as a “universal system” for diagnostic ECGs and for monitoring electrocardiographic applications.
Methods
Eighty women and men (27 to 91 years of age) were included. Of these, 42 had normal electrocardiographic waveforms (23 women and 19 men), 18 had left QRS axis deviation (10 women and 8 men), and 25 had QRS changes of inferior MI (10 women and 15 men) on their standard electrocardiographic recordings. Five patients had left QRS axis deviation and inferior MI. Patients with left QRS axis deviation were included to provide a wide range of QRS axes for comparing M-L and LU waveform deviations from standard. Inferior infarcts were identified by significant inferior Q waves or Q-equivalent patterns according to the Glasgow electrocardiographic interpretation program. The other ECGs were also assessed according to this program. The noise immunity substudy included 11 patients from the original cohort (2 women and 9 men, 59 to 75 years of age) and 9 healthy volunteers (6 women and 3 men, 25 to 58 years of age).
All patients were admitted to the Lund University Hospital (Lund, Sweden) during 2008 or 2009, to the department of clinical physiology, the cardiac intensive care unit, or the medical emergency care unit. One of the authors (AW) searched in the Infinity Megacare electrocardiographic server (Draeger Medical, Telford, Pennsylvania) for patients who had ECGs meeting the study requirements and then enrolled them at the 2 nursing wards. At the department of clinical physiology, electrocardiographic recordings were performed by 2 technicians. Patients were included in the noise immunity substudy whenever possible, but to attain a larger study group, 9 healthy volunteers at the department of clinical physiology were also enrolled.
The ethics committee at Lund University made an advisory statement in which it considered the study to be valuable and that there were no objections against it from an ethics point of view.
At the department of clinical physiology and at the cardiac intensive care unit, electrocardiographic recordings were made with a Megacart recording device (Siemens-Elema AB, Solna, Sweden). At the medical emergency care unit, a MAC 5500 recording device (GE Healthcare, Milwaukee, Wisconsin) was used. In the 2 recording devices, electrocardiographic signals were stored with a sampling interval of 2 ms.
For each of the 80 patients in the morphologic study, 4 ECGs were recorded within a few minutes of each other—2 standard ECGs, 1 LU ECG, and 1 M-L 12-lead ECG. Positions of the distal limb electrodes were unchanged between the 2 standard recordings. For LU ECGs arm electrodes were placed proximally and laterally on the arms and the left leg electrode proximally on the femoral bone; for M-L ECGs arm electrodes were placed on the lateral subclavicular fossae and the left leg electrode on the torso ( Figure 1 ). For the 2 alternative electrode-placement systems, chest electrodes remained in the same positions as for the standard 12-lead ECGs. Electrocardiographic recordings were made at rest with the patient in a supine position.
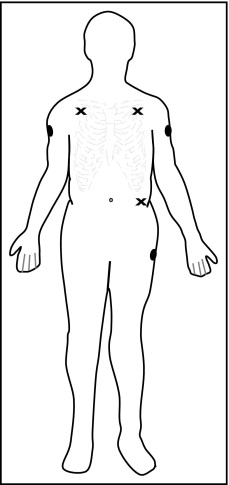
For each of the 20 subjects in the noise immunity substudy, 2 standard, 2 LU, and 2 M-L 12-lead ECGs were recorded while subjects, in a supine position, performed 2 different limb movements known to influence the technical quality of the electrocardiographic signal. For each electrode-placement system, 2 recordings were made in sequence. During the first recording, subjects moved their legs up and down along the mattress; during the second recording, subjects performed “combing and shaving” movements with their arms and hands. Subjects began the movements a few seconds before the 10-second electrocardiographic recording began, to ensure that noise generated by the limb movements would be present on the analyzed ECG.
All ECGs were reanalyzed in the Infinity Megacare electrocardiographic server (Draeger Medical), which uses a Draeger version (number 24) of the Glasgow program to process ECGs. This processing generates several hundred measurements, including waveform measurements for each electrocardiographic lead. For all 80 patients, we chose the QRS axis in the frontal plane for comparative analysis. We performed Selvester scoring for all patients except for those 5 who had electrocardiographic findings that are considered confounding factors for QRS scoring (left ventricular hypertrophy or left anterior fascicular block). One patient who had QRS changes of anterior MI and 1 patient who had QRS changes of multiple MIs were also excluded. The automated measurements used for the QRS scoring were transferred to an EXCEL sheet (Microsoft, Redmond, Washington).
The Selvester scoring system was used to estimate the size of MI as percent left ventricle by quantitative evaluation of QRS changes on standard 12-lead ECG ( Figure 2 ). Each point has been designed to represent infarction of about 3% of the left ventricle. Infarct sizes assessed by the scoring system have been shown to correlate significantly with anatomically measured sizes of single MI in the anterior, inferior, and posterolateral thirds of the left ventricle. Scores from the 48 patients without QRS changes of MI were evaluated, as were scores from the 25 patients with QRS changes of inferior MI. The final score was counted only if the ECG complied with screening criteria of Anderson et al.
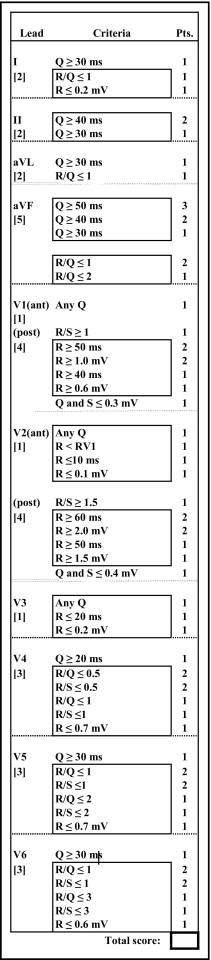
The Selvester scoring was based on the Glasgow program waveform measurements and calculated by 1 of the authors (AW). Another author (OP) scrutinized all QRS waveforms and automated measurements to possibly discover any mismeasurements of waveforms. A third author (GW) performed Selvester scoring based on visual/manual measurements. The group of investigators determined “adjudicated” scores in conference. No QRS measurements were changed in this process, but some Selvester scores were.
Differences in measurements between standard and LU ECGs and between standard and M-L ECGs were compared with differences between the 2 standard ECGs. For each patient, the first standard 12-lead ECG was used as the “gold standard” for comparison to all other ECGs.
Three electrocardiographic readers (2 physicians and 1 electrocardiographic technician) made subjective assessments of the noise immunity on each of the 6 ECGs obtained from each of the 20 subjects. Printouts of the ECGs did not indicate which lead system was used, and ECGs were submitted to readers in random order. Readers used a scale of 1 to 5 to indicate how much they thought the limb movements influenced the technical quality of the electrocardiographic signal, with 1 = very little influence, 2 = little influence, 3 = moderate influence, 4 = pronounced influence, and 5 = very pronounced influence. Readers assessed the total noise influence from limb movements, namely myoelectric noise from muscle activity and baseline wander from variations in electrode impedance mainly caused by the physical activity. Influence levels 1 and 2 were combined for analysis, as were influence levels 3 to 5, because we assumed that influence levels 1 and 2 should not influence diagnostic accuracy, whereas influence levels 3 to 5 might. When evaluating the data, assessments from the 3 readers were combined.
Paired t test was used for comparison of QRS axis variables obtained with each method, Wilcoxon signed-rank test was used for MI size variables, and chi-square statistic was used to compare categories of noise-immunity variables.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


