Patients with obstructive hypertrophic cardiomyopathy (HC) have various left ventricular (LV) shapes: reverse septal curvature (RSC, commonly familial), sigmoid septum (SS, common in hypertensives), and concentric hypertrophy (CH). Longitudinal (systolic and early diastolic) strain rate (SR) is sensitive in detecting regional myocardial dysfunction. We sought to determine differences in longitudinal SR of patients with obstructive HC, based on LV shapes. We studied 199 consecutive patients with HC (50% men) referred for surgical myectomy. Clinical and echocardiographic parameters were recorded. LV shapes were classified on echocardiography, using basal septal 1/3 to posterior wall ratio: RSC = ratio >1.3 (extending to mid and distal septum), SS = ratio >1.3 (extending only to basal 1/3), and concentric = ratio ≤1.3. Longitudinal systolic and early diastolic SRs were measured from apical 4- and 2-chamber views (VVI 2.0; Siemens, Erlangen). Distribution of RSC, SS, and CH was 50%, 28%, and 22%, respectively. Patients with RSC were significantly younger (47 ± 12 vs 64 ± 10 and 57 ± 11, respectively) with lower hypertension (40% vs71% and 67%, respectively) than patients with SS or CH (both p <0.001). Patients with RSC had lower global systolic (−0.99 ± 0.3 vs −1.05 ± 0.3 and −1.17 ± 0.3) and early diastolic SR (0.95 ± 0.4 vs 0.98 ± 0.3 and 1.16 ± 0.4) versus patients with SS and CH (in 1/s, both p <0.01), despite being much younger and less hypertensive. RSC was associated with abnormal global LV systolic (beta 0.16) and early diastolic (beta −0.17) SR (both p <0.01). In conclusion, patients with HC with RCS have significantly abnormal LV mechanics, despite being younger and less hypertensive. A combination of LV mechanics and shapes could help differentiate between genetically mediated and other causes of obstructive HC.
A variety of echocardiography-based techniques have been utilized to assess global and regional left ventricular (LV) mechanics (strain and strain rate [SR]), in patients with hypertrophic cardiomyopathy (HC) and various other disorders. Although the phenotypic expression of hypertrophy, obstruction, and diastolic dysfunction are typically progressive in HC, there is increasing recognition that there are changes in regional LV function (both systolic and diastolic) that predate overt morphologic and functional LV changes. Recently, speckle tracking echocardiography (STE) has emerged as an accurate and sensitive tool to study global and regional LV mechanics. There are no data describing differences in LV myocardial mechanics and deformation, based on the morphologic shape of the LV and ventricular septum, in patients with HC. We hypothesized that patients with reverse septal curvature (RSC, more likely to represent genetically mediated form of HC) would have worse global and regional LV mechanics, despite having similar degree of LV outflow obstruction, compared with sigmoid septum (SS) and concentric hypertrophy (CH; other morphologic subtypes seen in HC). We sought associations of systolic and diastolic LV mechanics, measured by STE, with the different morphologic shapes in adult patients with HC with similar degree of symptomatic LV outflow obstruction who underwent surgical myectomy.
Methods
The study population consisted of 199 consecutive patients with HC with severe LV outflow obstruction who underwent surgical myectomy at our tertiary referral center. We excluded patients with fixed obstruction (subaortic membrane or aortic stenosis) that also had a concomitant myectomy, as these patients have a different pathophysiologic profile. To maintain homogeneity of the study population, in terms of afterload, LV outflow obstruction, and gradients, we only selected patients with severe LV outflow obstruction who underwent surgical myectomy. An initial clinical diagnosis of HC was established after a thorough history, examination, electrocardiography, and imaging. HC was defined as a hypertrophied and nondilated left ventricle in the absence of another cardiac or systemic disease that could result in a similar magnitude of hypertrophy. All patients had symptoms attributable to severe dynamic LV outflow obstruction, despite optimal medical therapy at maximum tolerated dosages and were referred for surgical intervention after consensus between cardiologists and cardiothoracic surgeons. Baseline demographic, clinical, and imaging data were collected. The study patients are part of an institutional review board–approved registry.
All patients underwent comprehensive echocardiograms using commercially available instruments (HDI 5000; Philips Medical Systems, N.A., Bothell, Wash and Acuson Sequoia; Siemens Medical Solution USA, Inc., Malvern, Pennsylvania) as part of standard workup. End-diastolic interventricular septal and posterior wall thickness was measured in a standard fashion according to the guidelines. LV ejection fraction and volumes were measured, using the standard short-axis, 2-, and 4-chamber views. LV volumes were subsequently indexed to body surface area. Resting LV outflow peak velocity was measured by a continuous-wave Doppler echocardiography, and resting LV outflow pressure gradient was estimated using simplified Bernoulli equation. Care was taken to avoid contamination of the LV outflow waveform by mitral regurgitation jet. In patients with resting left ventricular outflow tract gradients <30 mm Hg, provocative maneuvers, including Valsalva, amyl nitrite, and exercise echo, were also used to measure a provocable LV outflow gradient. In patients with resting peak LV outflow gradient >100 mm Hg, provocative maneuvers were not used. Maximal LV outflow gradient was recorded and defined as the highest recorded gradient (resting or provoked) in a given patient. In addition, resting afterload was roughly estimated as the sum of resting LV outflow gradient + systolic blood pressure (mm Hg). Degree of resting mitral regurgitation was assessed by color Doppler and quantified according to the multiple established criteria, on a scale of 0 to 4+ (0 = none, 1+ = mild, 2+ = moderate, 3 = moderately severe, and 4+ = severe). Grades of diastology were assigned.
Using various end-diastolic long- and short-axis echocardiographic views, all patients were classified into 3 categories, based on the morphologic shape of the septum: RSC, concentric hypertrophy (CH), and SS ( Figure 1 ). Initially, qualitative visual classification of the LV shape in asymmetric septal hypertrophy patients was performed as follows. RSC was defined as a predominant midseptal convexity toward the LV cavity, with the cavity having a crescentic shape. SS was defined as a generally ovoid cavity with the septum being concave toward the LV with a pronounced basal septal bulge. CH was visually identified as similar degree of hypertrophy throughout the LV. In addition to the above qualitative assessment, a quantitative assessment was made as follows: every patient was initially classified as having asymmetric septal versus CH, based on the ratio of septum to posterior wall. Asymmetric septal hypertrophy was considered if the ratio of septum to posterior wall was ≥1.3, although CH was considered if that ratio was <1.3. Subsequently, patients with asymmetric septal hypertrophy were further classified into those with RSC versus SS as follows: on end-diastolic 4-chamber image, the septum was divided into basal, mid, and apical 1/3, and if the hypertrophy (ratio of septum to posterior wall ≥1.3) was present only in the basal segment, the patient was classified as having SS. In contrast, if the hypertrophy (ratio of septum to posterior wall ≥1.3) extended to the basal and mid portions, the patient was classified as having RSC. This analysis was independent and blinded to all other analyses.
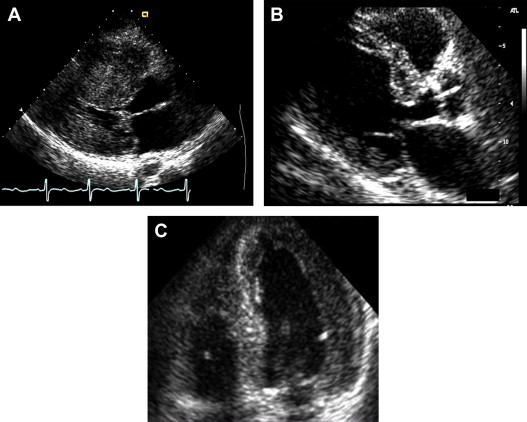
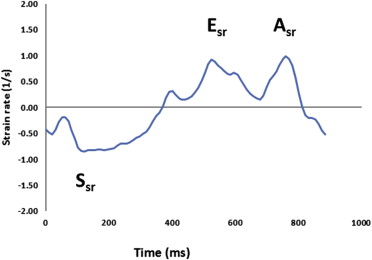
Subsequently, longitudinal strain and SRs (systolic and early diastolic) were measured in 2-chamber and 4-chamber views in an off-line manner using STE (40 frames/s, Velocity Vector Imaging, version 2.0; Siemens, Erlangen, Germany). In each view, endocardial borders were manually traced in the end-systolic frame, with the software subsequently automatically tracking myocardial deformation ( Figure 2 ). If poor tracking occurred, endocardial borders were readjusted manually until satisfactory tracking was achieved. Strain and SR curves for each segment (summed average) were then generated automatically. These measurements were made at the apical, mid, and basal levels of all visualized LV walls, and composite global strain and SR values for every patient were calculated. We wanted to understand the association of regional LV mechanics during both systole and diastole. As a result, we utilized SR, as opposed to strain. Additionally, there was a highly significant agreement between systolic strain and SRs. STE analysis was also blinded from all other analyses. All STE and LV shape analyses were re-performed in 10 randomly selected patients to assess reproducibility.
Patients underwent surgical myectomy in a manner previously described. In addition, patients who had persistent left ventricular outflow tract obstruction despite an adequate myectomy also underwent concomitant mitral valve or papillary muscle reorientation surgery. Patients with significant obstructive coronary artery disease underwent concomitant coronary bypass grafting.
Baseline demographics, risk factors, and clinical variables were summarized for the group. Continuous variables are expressed as a mean ± SD. Categorical data are presented using percentage frequencies. Differences between groups were determined using Student t test or analysis of variance for continuous variables (Mann-Whitney test for nonparametric variables) and the chi-square test for categorical variables. Reproducibility of SR measurements was assessed using intraclass correlation coefficient. Reproducibility of LV shape classification was assessed using kappa statistic. Regression analysis was performed in 3 steps. Initially, univariable regression analysis was performed to test the association between dependent variable (either systolic or early diastolic longitudinal SR) and various standard predictors. Subsequently, multivariable regression analysis was performed using predictors that had a p value <0.10 on univariable analysis. As a final step, the predictors that remained significant in step 2 were entered into an analysis of covariance model, along with 3 LV shapes, to test for association with the dependent variable (either systolic or early diastolic longitudinal SR). A p value <0.05 was considered significant. Data assembly and statistical analysis were performed with SPSS version 19.
Results
The present study population was relatively young with a mean age of 54 ± 13 years, with 50% men. All patients were on maximally tolerated medications at the time of surgery and were intractably symptomatic. Although 55% patients had a diagnosis of hypertension, it was classified as mild in most cases and not deemed severe enough to cause the degree of LV hypertrophy and resultant LV outflow obstruction. The clinical data are listed in Table 1 .
| Variable | Total Population (n = 199) | Reverse Septal Curvature (n = 100) | Sigmoid Septum (n = 56) | Concentric Hypertrophy (n = 43) | p Value |
|---|---|---|---|---|---|
| Age (yrs) | 54 ± 13 | 47 ± 12 | 64 ± 10 | 57 ± 11 | <0.001 |
| Male gender | 99 (50) | 49 (50) | 28 (50) | 22 (50) | 0.9 |
| Hypertension | 109 (55) | 40 (40) | 40 (71) | 29 (67) | <0.001 |
| Diabetes mellitus | 19 (10) | 8 (8) | 5 (9) | 6 (14) | 0.4 |
| Smoker | 65 (33) | 31 (32) | 19 (34) | 15 (35) | 0.9 |
| Significant CAD | 25 (13) | 7 (7) | 16 (29) | 2 (5) | <0.001 |
| Atrial fibrillation | 15 (8) | 6 (6) | 6 (11) | 3 (7) | 0.6 |
| Syncope | 46 (23) | 23 (12) | 11 (6) | 12 (6) | 0.7 |
| β-blocker use | 163 (82) | 80 (80) | 43 (77) | 40 (93) | 0.09 |
| QRS duration on electrocardiogram >120 ms | 7 (12) | 7 (7) | 10 (18) | 7 (16) | 0.09 |
The echocardiographic data of the 3 subgroups, including strain analysis, are listed in Table 2 . All patients had preserved LV ejection fraction, and no patient had abnormal LV chamber dilatation. All patients had severe LV hypertrophy (maximal LV thickness 2.1 ± 0.5 cm) and left ventricular outflow tract obstruction (maximal LV outflow gradient of 102 ± 35 mm Hg). Within the study population, 73% had abnormal relaxation, 20% had pseudo normal, and 7% had restrictive diastology. The echocardiographic data of the 3 subgroups are listed in Table 2 .
| Variable | Total Population (n = 199) | Reverse Septal Curvature (n = 100) | Sigmoid Septum (n = 56) | Concentric Hypertrophy (n = 43) | p Value |
|---|---|---|---|---|---|
| LV ejection fraction (%) | 62 ± 6 | 62 ± 5 | 62 ± 6 | 63 ± 6 | 0.8 |
| Indexed LV end-systolic volume (ml/m 2 ) | 21 ± 11 | 19 ± 10 | 21 ± 11 | 20 ± 10 | 0.3 |
| Indexed LV end-diastolic volume (ml/m 2 ) | 51 ± 21 | 50 ± 20 | 53 ± 22 | 51 ± 21 | 0.4 |
| Degree of mitral regurgitation (0–4+) | 1.8 ± 0.9 | 1.7 ± 1 | 1.9 ± 0.9 | 2 ± 1.2 | 0.2 |
| Maximal LV outflow gradient (mm Hg) | 102 ± 35 | 99 ± 36 | 101 ± 28 | 109 ± 38 | 0.3 |
| Afterload (mm Hg) | 185 ± 52 | 178 ± 48 | 184 ± 58 | 201 ± 50 | 0.05 |
| Basal septal thickness (cm) | 2.1 ± 0.5 | 2.4 ± 0.5 | 2.0 ± 0.3 | 1.8 ± 0.3 | <0.001 |
| Left atrial volume (ml) | 57 ± 29 | 58 ± 31 | 56 ± 25 | 56 ± 27 | 0.9 |
| E/A ratio | 1.31 ± 0.5 | 1.38 ± 0.7 | 1.26 ± 0.8 | 1.20 ± 0.5 | 0.4 |
| E/E′ ratio | 15 ± 8 | 14 ± 8 | 15 ± 6 | 17 ± 6 | 0.3 |
| Deceleration time (ms) | 255 ± 85 | 260 ± 97 | 255 ± 65 | 245 ± 81 | 0.6 |
| Color m-mode propagation velocity (m/s) | 87 ± 54 | 86 ± 43 | 98 ± 45 | 76 ± 32 | 0.4 |
| Basal septal systolic SR (1/s) | −0.92 ± 0.4 | −0.82 ± 0.4 | −1.01 ± 0.5 | −1.06 ± 0.5 | 0.003 |
| Basal septal early diastolic SR (1/s) | 0.82 ± 0.4 | 0.73 ± 0.4 | 0.83 ± 0.5 | 1.01 ± 0.6 | 0.004 |
| Global longitudinal systolic SR (1/s) | −1.04 ± 0.3 | −0.99 ± 0.3 | −1.05 ± 0.3 | −1.17 ± 0.3 | 0.004 |
| Global longitudinal early diastolic SR (1/s) | 1.00 ± 0.4 | 0.95 ± 0.4 | 0.98 ± 0.3 | 1.16 ± 0.4 | 0.008 |
The reproducibility of longitudinal septal SR analysis for our study population was ascertained in a group of 10 randomly selected patients. For systolic SR analysis, the intraclass correlation coefficient for intraobserver (MD) and interobserver (MD and TK) reproducibilities was 0.94 (0.79 to 0.98) and 0.88 (0.74 to 0.98), respectively. Similarly, for early diastolic SR analysis, the intraclass correlation coefficient for intraobserver (MD) and interobserver (MD and TK) reproducibilities was 0.93 (0.73 to 0.98) and 0.86 (0.56 to 0.96), respectively. The intraobserver (MD) and interobserver (MD and AD) kappa values for LV shape assessment were 0.89 and 0.87, respectively.
In steps 1 and 2, using global longitudinal systolic and early diastolic SRs as dependent variables, we tested their association with various potential clinical and imaging predictors, using univariable and multivariable regression analysis ( Tables 3 and 4 ). In the third step, we performed analysis of covariance to test the association between global systolic and diastolic SR and various LV shapes (RSC, SS, and CH), also including (as covariates) the factors that were significant on multivariate analysis from Tables 3 and 4 . The results are listed in Table 5 . As listed in Table 5 , patients with HC with RSC, despite being significantly younger had a significant association with abnormal global systolic and early diastolic SRs, independent of other covariates. In contrast, SS had no independent association with SRs.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Beta | p Value | Beta | p Value | |
| Age (yrs) | −0.03 | 0.6 | ||
| Gender | −0.18 | 0.01 | −0.19 | 0.005 |
| Hypertension | 0.084 | 0.2 | ||
| Diabetes mellitus | 0.05 | 0.5 | ||
| Smoking | −0.04 | 0.6 | ||
| Obstructive coronary artery disease | 0.21 | 0.08 | 0.08 | 0.2 |
| QRS duration on electrocardiogram >120 ms | 0.04 | 0.5 | ||
| β blockers | 0.20 | 0.005 | 0.22 | 0.001 |
| LV ejection fraction (%) | 0.04 | 0.7 | ||
| Maximal LV thickness (cm) | 0.21 | 0.004 | 0.21 | 0.002 |
| Indexed LV end-systolic volume | 0.05 | 0.2 | ||
| Indexed LV end-diastolic volume | 0.04 | 0.7 | ||
| Resting LV outflow tract gradient (mm Hg) | 0.04 | 0.6 | ||
| Maximal LV outflow gradient (mm Hg) | −0.006 | 0.9 | ||
| LV afterload | −0.03 | 0.7 | ||
| Left atrial volume | 0.11 | 0.2 | ||
| E/A ratio | −0.002 | 0.9 | ||
| E/E′ ratio | 0.11 | 0.2 | ||
| Color m-mode propagation velocity | −0.07 | 0.5 | ||
| Deceleration time | 0.06 | 0.4 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


