14 Diastolic Dysfunction and Echocardiographic Hemodynamics
Basic Equations and Their Assumptions
Doppler Shift Equation
where V = blood velocity, Fd = Doppler shift, cosθ = cosine of the Doppler angle, and K= speed of ultrasound in tissue 2× carrier frequency.
Bernouilli Equation
where P = density of fluid, ds = the “path element,” V = velocity, ΔP = pressure drop across the orifice, and R = resistance due to viscous friction. V1 is usually much less than 1.5 m/sec and is generally ignored.
Three factors that affect pressure drop across an orifice are
Diastolic Function, Dysfunction, and Heart Failure with Normal Ejection Fraction
The Four Phases of Diastole
The IVRT is calculated from aortic valve closure to mitral valve opening. IVRT is therefore
Hence, the E-wave is not synonymous with myocardial relaxation.
 LA contractility: In advanced congestive heart failure, the LA contribution to LV filling decreases as LA systolic function fails.1
LA contractility: In advanced congestive heart failure, the LA contribution to LV filling decreases as LA systolic function fails.1
 The left ventricular end-diastolic pressure (atrial afterload)
The left ventricular end-diastolic pressure (atrial afterload)
 RV forces: In patients with RV dilation and RV systolic pressures >40 mm Hg, there is increased atrial systolic filling of the LV.2
RV forces: In patients with RV dilation and RV systolic pressures >40 mm Hg, there is increased atrial systolic filling of the LV.2
Diastolic Function, Dysfunction, and Heart Failure
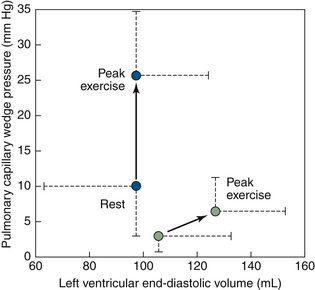
The function of diastole is to provide adequate volume load to the ventricles for the systolic output needs, at filling pressures physiologically acceptable to the atria and venous drainage. Traditionally, diastolic properties of the left ventricle were held to be the principal determinant of exertional tolerance in cases of systolic dysfunction. “Diastolic dysfunction” is a variably used term, but generally means that the pattern and properties of diastolic filling are abnormal, reflecting abnormal diastolic properties. Exercise intolerance in patients with HFNSF is attributed to failure of the Frank-Starling mechanism (Fig. 14-1).
Diastolic heart failure is the clinical manifestation of severe diastolic dysfunction occurring in the absence of systolic dysfunction (usually ejection fraction [EF%] >55, although definitions differ). “Diastolic heart failure” has become a controversial term, increasingly replaced by “heart failure with normal systolic function” (HFNSF)—a term that is solely descriptive and does not speculate about the etiology.3 In the absence of systolic dysfunction, the presence of heart failure (after excluding valvular disease and coronary artery disease) is not invariably due to diastolic failure, as volume excess may be the principal cause of heart failure.
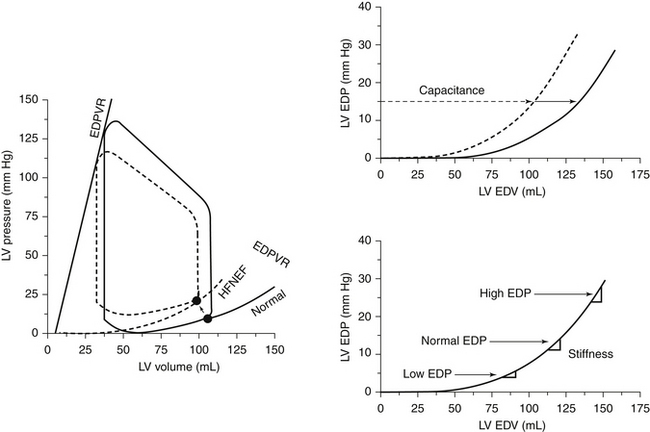
The most accurate means by which to evaluate diastolic dysfunction of the heart has evolved over decades, to become increasingly complex and invasive, rather than less complex and invasive. Hemodynamic studies that describe the stiffness of the heart through the end-diastolic pressure volume relationship (EDPVR), by reducing the venous inflow to the heart and simultaneously measuring the diastolic pressure and volume in the left ventricle with a conductance catheter, are held to be the best means to establish diastolic failure of the heart. A stiff ventricle has an upward-shifted EDPVR.3 The complexity and logistics of this catheterization technique simply prohibit its routine use. The findings of patients with HFNSF are upshifted EDPVR at reduced, normal, or even increased volumes (Figs. 14-2 and 14-3).3 No single adequate explanation of HFNSF exists, and the topic is becoming more, not less, complicated.
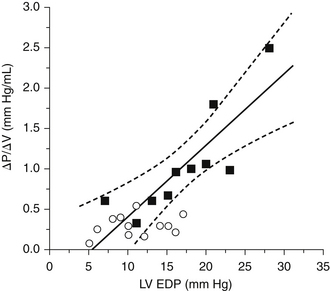
Figure 14-3 Data re-plotted from Table 1 of Grossman et al.24 showing that diastolic stiffness (∆P/∆V) varies directly with filling pressure. Data from 10 hypertrophic hearts (solid squares) span high filling pressures and stiffness values. Data from one patient of the original publication with aberrant data (end-diastolic pressure [EDP] of 52 mm Hg and stiffness of 0.9 mm Hg/mL) was excluded. Line of linear regression (±95% confidence interval) was determined for data from these hypertrophic hearts. Data from 12 nonhypertrophic patients (open circles) have lower filling pressures and lower values of stiffness; a majority of these data fell within the 95% confidence intervals of the regression line determined from the patients with hypertrophy.
(From Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107:656–658.)
LV inflow, pulmonary venous, indicies, and velocity of propagation Doppler echocardiography indices of diastolic dysfunction have not provided the same precision or the correlation to clinical status that catheter indices provided. LV inflow and pulmonary venous patterns are influenced by volume effects. Tissue Doppler indices, which may be afterload influenced, appear to offer more correlation. Many individuals have abnormal Doppler patterns of diastolic filling, but do not have heart failure. The Doppler diastolic parameters of patients with HFNSF are variable.4
 Conventional Doppler diastolic indicies correlate only moderately with catheter-derived indicies of diastolic failure5:
Conventional Doppler diastolic indicies correlate only moderately with catheter-derived indicies of diastolic failure5:
• E/A: r = –0.36; IVRT: r = 0.31; E´/A´lateral (<1): r = –0.37; E/E´lateral (≥8): r = 0.53*
 Diastolic dysfunction is detected in only 70% of patients by LV inflow patterns, but in 81% of cases by E´/A´lateral, and in 86% of cases by E/E´lateral.
Diastolic dysfunction is detected in only 70% of patients by LV inflow patterns, but in 81% of cases by E´/A´lateral, and in 86% of cases by E/E´lateral.
 Using either E´/A´lateral or E/E´lateral detected 93% of cases.
Using either E´/A´lateral or E/E´lateral detected 93% of cases.
 In all patients LV inflow profiles and tissue Doppler are suitable for analysis, but only 60% of pulmonary venous profiles are suitable.5
In all patients LV inflow profiles and tissue Doppler are suitable for analysis, but only 60% of pulmonary venous profiles are suitable.5
Vasan and Levy6 proposed criteria for diagnosing diastolic heart failure/HFSNF:
 Definite diastolic heart failure/HFSNF
Definite diastolic heart failure/HFSNF
• Definitive clinical evidence of heart failure
• Objective evidence of normal systolic function (>50%) within 72 hours of the heart failure event
• Objective evidence of LV diastolic dysfunction on catheterization (abnormal LV relaxation/filling/distensibility)
 Probable diastolic heart failure/HFSNF
Probable diastolic heart failure/HFSNF
• Definitive clinical evidence of heart failure
• Objective evidence of normal systolic function (>50%) within 72 hours of the heart failure event
• No conclusive objective evidence of LV diastolic dysfunction on catheterization (abnormal LV relaxation/filling/distensibility)
 Possible diastolic heart failure/HFSNF
Possible diastolic heart failure/HFSNF
• Definitive clinical evidence of heart failure
• Objective evidence of normal systolic function (>50%) outside of a 72-hour window of time from the heart failure event
• Objective evidence of LV diastolic dysfunction on catheterization (abnormal LV relaxation/filling/distensibility) is lacking
As can be seen, the only of these criteria that echocardiography contributes to is the punctual assessment of LV systolic function. Echocardiographic indices of diastolic function are not criteria according to Vasan and Levy, but are included in the European Study Group on Diastolic Heart Failure report.7
Most patients with HSNSF are elderly women with mild left ventricular hypertrophy and chronic advanced heart failure symptoms.8 Borderline or mildly increased wall thickness is usual, but severe left ventricular hypertrophy is unusual.4 The left atrium is almost invariably enlarged (volume appears superior to dimension); hence, a normal-sized left atrium can be held against the diagnosis of diastolic heart failure.9,10
Traditionally, echocardiography assesses diastolic function by three means:
1. Pulsed-wave analysis of traditional LV inflow patterns
2. Pulsed-wave analysis of pulmonary venous flow patterns
3. Tissue Doppler imaging of the septal and lateral mitral annuli
Diastolic Dysfunction Categories: Nomenclature
 Impaired relaxation, also known as
Impaired relaxation, also known as
 Restrictive reversible, also known as
Restrictive reversible, also known as
 Restrictive fixed, also known as
Restrictive fixed, also known as
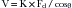
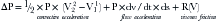





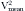

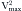 , where Vmax = peak velocity
, where Vmax = peak velocity