Parameter
Cutoff
Cutoff
Absolute values
PF/Sa – Ratio
Transudate↓
Transudate↓
Exudate↑
Exudate↑
Protein
3 g/dl
0.5
Lactic dehydrogenase (LDH)
200 IU/dlb
0.6
Cholesterol
60 mg/dlc
In the diagnosis of exudative etiologies, apart from its classical role in malignancy, thoracoscopy is also an excellent tool for the diagnosis of various benign diseases. The etiologic spectrum and relative frequency of benign exudative effusion, i.e., mostly inflammatory pleural disease, are summarized in Table 11.2,in which bacterial pleurisy (parapneumonic effusion and empyema) is clearly the main cause (Light 1995a). Tuberculous pleurisy has become rare in Western populations but remains a crucial issue in light of globalization and in developing countries, particularly in the context of the HIV epidemic. Nonspecific pleurisy is caused by different conditions such as thromboembolism and rheumatological disease that are often clinically underestimated and also by a variety of difficult-to-diagnose inflammatory conditions, which may have anecdotic importance only, for example, benign asbestos. Nevertheless, thoracoscopy may also play an important role in some of these conditions. The diagnostic value and overall role of thoracoscopy in the management of various benign pleural effusions will be discussed in this chapter.
Table 11.2
Etiologic spectrum and causes of exudative pleural effusion and their relative incidence
Etiology | Incidence (%) |
|---|---|
Pneumonia | 50.9 |
Pleural malignancies | 25.5 |
Thromboembolism | 18.0 |
Gastrointestinal disease | 4.0 |
Rheumatic-autoimmunologic disease | 0.8 |
Tuberculosis | 0.55 |
Benign asbestos pleuritis | 0.25 |
11.2 Parapneumonic Effusion and Empyema
11.2.1 Clinical Background
Parapneumonic effusion and empyema are interrelated, overlapping manifestations of bacterial pleurisy. The most common cause, pneumonia, is present in about 55 % of cases, while bacterial pleurisy complicates pneumonia in 20–57 % of cases. Thus in the majority of cases, the condition presents as pleuropneumonia (Alfageme et al. 1993). Bacterial pleurisy is estimated to account for 20–30 % of all cases of pleural effusion. Serious and debilitating predisposing conditions are observed in up to 82 % of cases, of which compromised immunity due to alcoholism is the single biggest risk factor (Alfageme et al. 1993). The effusion is unilateral in 80 % of cases and may be profuse or sometimes even lead to intrathoracic displacement and compression. The most important differential diagnosis relevant to local treatment is lung abscess, which, however, will not usually require thoracostomy. Contrast-enhanced CT is helpful for making this distinction using the criteria presented in Table 11.3.
Table 11.3
Differentiation between empyema and lung abscess on a (contrast) CT scan
Signs favoring empyema |
Signs of lung compression |
Smooth margins of membranes |
Dissection of the thickened visceral parietal pleura |
Blunt angle with the chest wall |
Signs favoring abscess |
Spherical shape with irregular and thicker wall structures |
Absence of lung compression |
Sharp angle with the chest wall |
Visible airway connection |
Demonstration of vasculature around abscess (definite proof) |
11.2.2 Prethoracoscopic Investigations
The diagnosis of parapneumonic effusion may be straightforward in situations where the clinical background is compatible and the culture is positive, foul smelling turbid effusion has been recovered, or frank empyema is found at thoracocentesis. In the presence of serous clear pleural fluid and negative microbiological findings, further investigations are needed to establish a diagnosis of bacterial pleurisy. Light’s criteria, including pH, glucose content, and LDH, are then commonly recommended for subclassification of bacterial pleurisy into uncomplicated and complicated parapneumonic effusions. Previously, these overlapping conditions were referred to as exudative or fibrinopurulent pleurisy and organizing or chronic empyema. As shown in Table 11.4, empyema and complicated parapneumonic effusion, which are classical high-dependency indications for thoracostomy and drainage, are characterized by low pH (<7.0) and glucose (<2.2 mmol/l (40 mg/dl)) and elevated lactate dehydrogenase (LDH) levels (>10,000 IU/l) and WBC count (>15 × 109/l), with a bacterial culture which is usually positive (Heffner et al. 1995; Maskell and Davies 2003). While uncomplicated effusion warranting noninvasive management is well defined, on the other hand, these criteria may leave a predictive gap for poorly defined intermediate effusions, as is also shown in Table 11.4. Based on the key parameter, pH, and a comprehensive body of evidence from individual studies and meta-analyses, current recommendations of the American College of Chest Physicians (ACCP) propose a more stringent differentiation into four risk and outcome categories (Davies et al. 2003; Antunes et al. 2003):
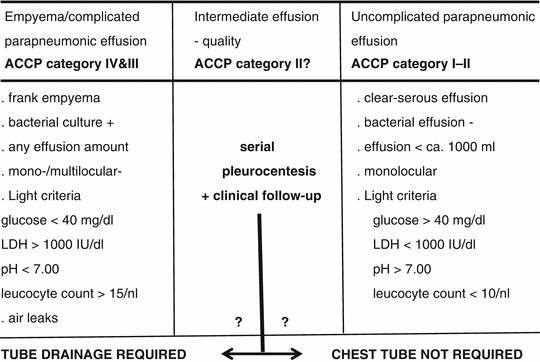
Table 11.4
Classification of parapneumonic effusion (bacterial pleurisy) and derived indications to tube drainage

Category I – minimal free-flowing effusion, culture and pH unknown
Category II – small to moderate free-flowing effusion, negative culture, and pH > 7.2
Category III – large free-flowing effusion, loculations and membranes, pH < 7.2
Category IV – category III plus evidence of frank pus
In broad practical terms, taking into account the clinical presentation and fluid characteristics, thoracostomy with tube drainage is therefore vitally important in one or more of the following conditions:
Frank empyema at thoracocentesis – ACCP category IV
Ongoing sepsis
Profuse effusion (>2 l), particularly where it causes displacement
Presence of air in the pleural space suggesting a bronchopleural fistula
Biochemical fluid parameters as defined in ACCP category III (usually termed “complicated parapneumonic effusion”)
11.2.3 The Role of Thoracoscopy
Any invasive approach that focuses on chest drainage clearly addresses and highlights the use of thoracoscopy (Colice et al. 2000; Loddenkemper and Frank 2006). The rationale of thoracoscopy in empyema and complicated parapneumonic effusion is mainly management related. The diagnostic value of biopsies taken under direct vision in neutrophilic inflammation of bacterial pleurisy is usually negligible – unless differential diagnoses such as tuberculosis or other specific etiologies are being considered.
The role of medical thoracoscopy in the management of empyema and parapneumonic effusion has long been under debate; this indication is only reluctantly accepted by pulmonologists as there is a paucity of evidence-based data and competitive surgical treatment options. Video-assisted thoracic surgery (VATS), in particular, has become a gold standard. For many years, experienced pulmonologists in Western Europe have routinely performed thoracoscopy in the setting of bacterial pleurisy and this has created a body of expertise. However, it remains largely on the evidence level of expert opinion. The “inventor” of medical thoracoscopy himself (Jacobaeus) was the first to report an extensive experience in 100 cases as early as 1925 (Jacobaeus 1925). Boutin and colleagues describe their good results in the preceding edition of this book (Boutin et al. 1991). In Israel, Weissberg successfully performed thoracoscopy in empyema in the early 1970s, using a mediastinoscope (Weissberg 1980). More recently, in a large retrospective series of 127 patients from various hospitals in Switzerland and Italy, all of whom presented with multiloculated empyema, medical thoracoscopy as the first-line intervention under local anesthesia was successful in 91 % of cases, with 94 % of patients being cured by nonsurgical means (Brutsche et al. 2005). In another series of 16 consecutive cases in whom medical thoracoscopy was performed after tube drainage had failed, clinical improvement was achieved in all cases and definitive cure in 12, thus only four cases required surgical debridement (Solèr et al. 1997). In America, Landreneau and colleagues also reported similarly successful use of thoracoscopy in 63 out of 99 patients (Landreneau et al. 1995). In pediatric cases, medical thoracoscopy performed under local anesthesia does not appear to be feasible, and thus video-assisted thoracic surgery (VATS) is considered the intervention of choice and given clear preference over formal thoracostomy (Colice et al. 2000; Davies et al. 2003; Antunes et al. 2003). Medical thoracoscopy is now also becoming increasingly accepted in English-speaking countries and has been adopted as a treatment option by many scientific societies. Surgical thoracoscopy (VATS) for the treatment of bacterial pleurisy is now firmly incorporated into the current guidelines and consensus statements of both the ACCP and the British Thoracic Society (BTS). According to the BTS guidelines, fibrinolysis, VATS, and formal surgery are equally acceptable interventions in empyema, but VATS is preferred to fibrinolysis (evidence level C) (Colice et al. 2000; Davies et al. 2003; Antunes et al. 2003). In this setting, medical thoracoscopy introduces the intriguing additional option of using a combined thoracoscopy/fibrinolysis approach which has the advantage of being only moderately invasive and more cost-effective. This concept is currently under investigation in a European multicenter project.
11.2.4 Procedure
The technical approach to performing a thoracoscopy in empyema is basically the same as that used for other types of effusion but, importantly, must specifically take into account loculations and membranes, which are typical for and frequently occur in empyema. A careful evaluation with CT and ultrasound, to define safe entry points, is mandatory prior to the thoracoscopy (Figs. 11.1 and 11.2) (Hersh et al. 2003). This is particularly important when sharp obturators are employed, in order to avoid injury to the lung. To define a safe entry compartment in a loculated effusion, an accessible liquid compartment must be firstly identified by fluid aspiration. In our opinion, this should be confirmed by fluoroscopy using gas insufflation (air or better CO2) to demonstrate gas deposition and thus lung detachment at the intended point of entry. Following entry into the pleural cavity, exploration may reveal widely varying inflammatory patterns.
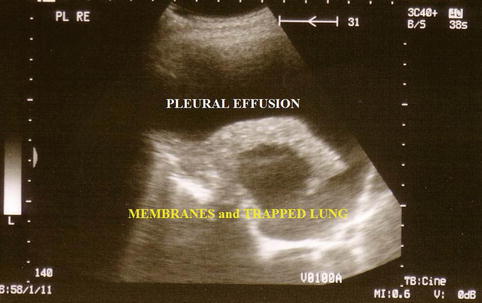
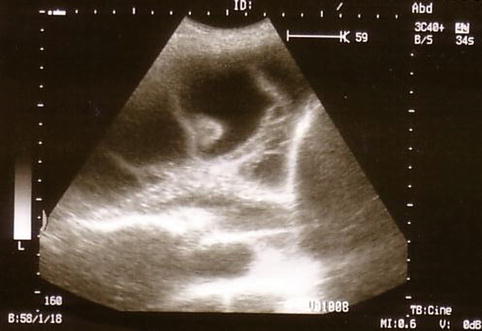

Fig. 11.1
Ultrasound to detect inflammatory visceral membranes and therefore a trapped lung secondary to empyema

Fig. 11.2
Ultrasound detection of a multiple loculated empyema
One typical macroscopic finding is the presence of an abundant, viscous accumulation of pus (of up to several liters) often in a monolocular cavity; the intensely inflamed parietal and visceral pleura is associated with purulent adherent membranes and hemorrhage, which may occur spontaneously. An example of such changes, which used to be referred to as the fibrinopurulent stage of empyema, is shown in Fig. 11.3.
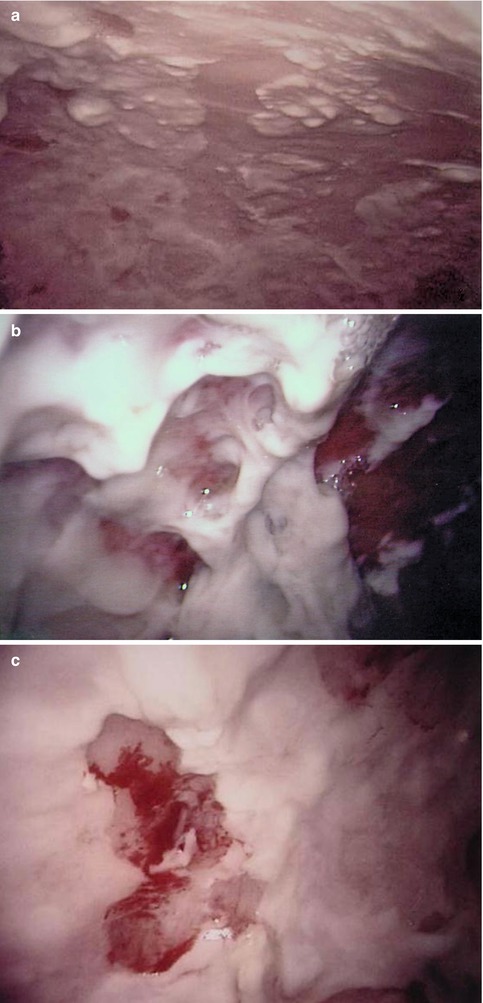
Fig. 11.3
Typical features of a single cavity secondary to empyema (parietal pleura, fibropurulent stage). Male, 48 years, purulent peels (a) including spontaneous hemorrhage (b) and biopsy lesion (to exclude TB) (c)
More often, as is typically seen in complicated parapneumonic effusions, the endoscopic finding is of multiple fibrinous loculations. The operator often visualizes adhesions and sponge-like chambers following the introduction of the telescope (Fig. 11.4). The investigation may then be expanded to include mechanical separation of compartments and blunt dissection of membranes and adhesions in order to create a large monolocular cavity which will subsequently be easier to treat. This procedure can also include peeling of thick visceral membranes, making it possible to free and re-expand a trapped lung. With these dissection procedures, particular care must be taken to spare adhesions that are already organized and vascularized. This may occur in cases where the clinical course is prolonged and in the setting of chronic empyema. A typical sequence is shown in Fig. 11.5, with initial visualization of extensive loculations which are then broken down (debridement) to reveal the originally entrapped lung.
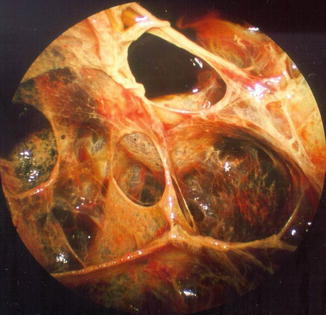
Fig. 11.4
The benefit of pleuroscopy in tube drainage management: recognition and elimination of multiloculation in empyema
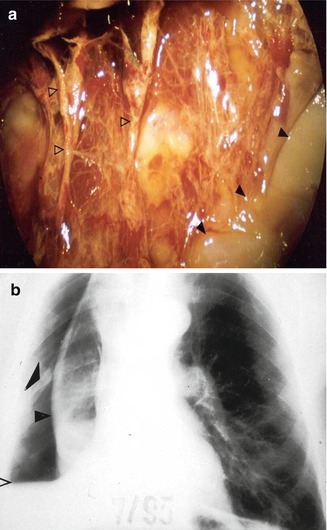
Fig. 11.5
The benefit of pleuroscopy in tube drainage management in empyema: same case as Fig. 11.4. Pleuroscopic features of the visceral membrane after dissection of the inflammatory adhesions. Black arrowheads pus deposits, white arrowheads visceral membrane (a). The visceral peel with indwelling chest tube preventing re-expansion of the trapped lung post pleuroscopy. Black arrowheads visceral peel (b). The lung subsequently expanded after fibrinolysis
11.2.5 Combined Treatment Modalities
Drain insertion is a vital procedure which is carried out in the acute phase of empyema, but it is also crucial for optimal long-term results. Another important advantage of thoracoscopy is that it allows visual control and thus also optimal drain placement. Drainage is performed not only to remove fluid but should also usually include irrigation with or without instillation of fibrinolytics. The drainage specifications should therefore give preference to large-bore, double-lumen catheters (≥24 F) to ensure clearance of thick secretions and initiation of closed circuit irrigation. If fibrinolysis is considered, streptokinase and urokinase are equally effective agents. According to several guidelines, the instillation of fibrinolytics is indicated in the presence of:
Fibrinolysis enhances the effects of interventional thoracoscopy and may obviate the need for two or more drains in multiloculated empyema. There will be a transient increase in fluid production due to the resolution and liquidation of pus and membranes. With irrigation and fibrinolysis protocols, the output of mostly turbid fluid will initially exceed the irrigation input. Irrigation is continued until a clear sterile fluid is obtained and the net fluid production falls below 50 ml/day. Our protocol has been well tried and proven in practice and uses a combination of streptokinase and streptodornase (DNAse) (see Table 11.5). The value of the streptodornase component has not yet been firmly established clinically; however, experimental data strongly suggest that the effectiveness of fibrinolysis can be improved by decreasing the DNA-dependent viscosity of the pus (Simpson et al. 2000). The only relevant contraindication to the use of streptokinase is suspected allergy. However, fibrinolytics may also be cautiously applied in the presence of bronchopleural fistula to avoid spilling and contamination of the ipsilateral and contralateral bronchial systems. Adverse effects of both streptokinase and urokinase may include fever >38.5 °C and pain (both up to 7 % of cases). Pleural fibrinolysis is safe as regards systemic effects, and there is no evidence that systemic fibrinolysis occurs at cumulative doses of up to six times the therapeutic level (Davies et al. 1998). A review of the evidence based on fibrinolysis from multicenter studies and meta-analyses reveals that 200,000–250,000 IU streptokinase was equally as effective as 50,000–100,000 IU urokinase (Bouros et al. 1997). Fibrinolysis was significantly more effective than pure irrigation as assessed on the basis of some secondary endpoints such as time to clearance of empyema and radiological resolution (Diacon et al. 2004; Maskell et al. 2005; Tokuda et al. 2006). There were no significant differences between the two procedures as regards the primary endpoints of mortality and surgery. Thus the benefits of fibrinolysis remain to some extent controversial and require further elaboration (Rahman et al. 2011).
Table 11.5
Combined irrigation/instillation protocol for fibrinolytic chest tube management of empyema (own protocol)
A: Interventional approach |
1. Ultrasound-guided thoracentesis |
2. Double-lumen irrigation/suction drainage (20–24 F, 40 cm), ultrasound guided pleuroscopy guided |
3. Empyema evacuation (standard suction: 20 cm H2O) |
4. Imaging control (X-ray/fluoroscopy/sonography) |
5. Irrigation using isotonic saline solution with aseptic additives (polyvidone iodine 2 %) until recovery of clear irrigation fluid (before and after fibrinolysis) |
6. Fibrinolytic agent instillation: Varidase® (1 ampule = 100,000 IE streptokinase + 25,000 IE streptodornase) with a 2–4 h clamping period |
B: Empiric and subsequent targeted antibiotic therapy |
11.3 Tuberculous Pleurisy
11.3.1 Clinical Background
Tuberculous pleural involvement may occur at any stage of the disease but is more likely to be associated with a primary pulmonary tuberculous infection. Most patients present with acute fever and the clinical picture mimics bacterial parapneumonic pleurisy, which is also the most important differential diagnosis. Pleuritis exsudativa tuberculosa is the classical term for this form of tuberculosis. The pleural fluid is serous and a high-grade exudate with a protein concentration usually greater than 5 g/dl. Pleural cellularity shows a prevalence of lymphocytes but may become transiently neutrophilic in the acute febrile and initial stages. Exudative pleuritis occurs unilaterally in the vast majority of cases and rarely, if ever, develops profuse or compressive features. Overt concomitant pulmonary involvement is often absent. Exudative pleuritis is unlike tuberculous empyema, which again may mimic nonspecific bacterial empyema. Tuberculous empyema is a sequel and complication of long-standing postprimary pulmonary tuberculosis that may be associated with the rupture of caseous pulmonary lesions, tuberculous fibrothorax, and calcareous pleural thickening.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


