Lung carcinoma
37 %
Breast carcinoma
16.8 %
Lymphoma
11.5 %
Genitourinary carcinomas
9.4 %
Gastrointestinal carcinomas
6.9 %
Other carcinomas
7.3 %
Unknown primary
10.7 %
Pleural effusion is usually diagnosed by a simple chest radiograph and/or computed tomography. In lung cancer, its presence is associated with advanced stage disease (M1a) and therefore with poor prognosis (Mountain 1997). However, in some cases, pleural effusion may be due to postobstructive pneumonia or atelectasis, venous obstruction by tumor compression, or lymphatic obstruction by mediastinal lymph nodes, and therefore it is not associated with direct pleural involvement (Antony et al. 2001). The confirmation of a benign pleural effusion in the setting of confirmed malignancy will have a major impact on treatment options and therefore prognosis. When pleural cytology is negative, thoracoscopy must be performed in order to evaluate the extent of the disease and confirm malignant infiltration of the pleura if present (Rodriguez Panadero 1995). Thoracoscopy also provides information about the extent of the disease especially in non-small-cell lung cancer (NSCLC) and mesothelioma. In NSCLC a positive thoracoscopy defines M1a disease, which excludes surgical resection and indicates a poor prognosis for the patient. In the case of a patient with NSCLC and pleural effusion, a negative thoracoscopy indicates a paramalignant effusion, and therefore the patient may be a candidate for radical surgery, with a presumed better prognosis. Thoracoscopy may have a role in early stage mesothelioma – the findings at the time of the procedure may suggest a role for multimodality treatments including extrapleural pneumonectomy. On the contrary, in a patient with known metastatic carcinoma or poor performance status (PS), establishing whether or not an effusion is malignant might not be necessary, since survival will be poor in either case.
9.2 Pathogenesis of Malignant Pleural Effusion
The occurrence of a pleural effusion in malignancy is usually secondary to obstruction of the lymphatic drainage from the pleural space, such as pleural thickening by widespread carcinomatosis, obstruction caused by infiltration of mediastinal lymph nodes, or obstruction caused by tumor emboli (Rodriguez-Panadero et al. 1989; Meyer 1966; Chernow and Sahn 1977). In addition a local inflammatory reaction to tumors next to the pleura might play an important role in the development of pleural effusions by increasing capillary permeability (Leff et al. 1978). These mechanisms explain the predominance of lymphocytes in malignant pleural effusion, although their role in the pathogenesis remains unclear. However, T lymphocytes might have an important role in host versus tumor local defense in malignant pleural effusions (Domagala et al. 1978).
The underlying mechanisms of pleural effusion due to neoplasms have been reported in postmortem studies (Rodriguez-Panadero et al. 1989; Meyer 1966). Most of the patients had both parietal and visceral pleura infiltration. It was shown that invasion of the parietal pleura is due to neoplastic spread across the pleural cavity from the visceral pleura along pleural adhesions which have been preformed or secondary to the malignant process. In addition, the parietal pleura is invaded by the attachment of exfoliated cells from the visceral pleura. The visceral pleura might be involved by direct pulmonary arterial invasion and embolization (Meyer 1966). When the tumor infiltrates blood vessels directly, and/or occludes venules, a bloody pleural effusion may occur. Another mechanism of hemorrhagic pleural effusion might be associated with capillary dilatation due to release of vasoactive substances (Meyer 1966; Chernow and Sahn 1977). Bilateral pleural metastases are due to hematogenous spread to the contralateral hemithorax (Meyer 1966).
Nonmalignant pleural effusions (paramalignant) may occur due to bronchial obstruction causing atelectasis or pneumonia with an associated parapneumonic effusion. In this situation the effusion is due to a mechanical obstruction without malignant cell infiltration of lymphatics or blood vessels. Lung carcinoma is also frequently associated with heart failure, as both bronchogenic carcinoma and heart disease have the same risk profiles. Thus, pleural effusion may be a transudate due to heart failure. The same finding may be observed in patients with bronchogenic carcinoma and hepatic insufficiency. In some patients there is an association between pulmonary embolism and pneumothorax (Kabnick et al. 1982) with bronchogenic carcinoma.
9.3 Clinical Presentation of Malignant Pleural Effusion
A pleural effusion in patients with neoplasm may present in two distinct clinical situations: as a presenting symptom, i.e., during the work-up a previously unknown carcinoma, or presenting as a pleural effusion during the evolution of a known carcinoma. The diagnostic approach, although basically the same, must be specific to each case as the patient’s prognosis is dependent on it. In the former, the pleural effusion is discovered after the patient consults his physician for progressively worsening dyspnea, dry cough, lateral thoracic pain, or hemoptysis. General constitutional symptoms may also be present, such as fever, weight loss, loss of appetite, and impairment of activities of daily living (ADLs). Only 25 % of patients are totally asymptomatic despite the presence of a pleural effusion on imaging studies. Typically there is evidence of a pleural effusion on the physical examination which is confirmed by chest X-ray, which also demonstrates the extent of the pleural process (Hyde and Hyde 1974; Cohen 1974; Scagliotti 1995).
9.4 Imaging
Pleural effusions may be free-flowing or loculated (White et al. 1993). The chest X-ray may also demonstrate the likely cause of the pleural effusion, such as a peripheral lesion in contact with the thoracic wall or a central lesion with associated atelectasis or obstructive pneumonia. It may also provide useful information on associated findings, such as pericardial effusion, air in the pleural space, or mediastinal lymph node enlargement (White et al. 1993; Romney and Austin 1990; Woodring 1990). In 15 % of cases however, the chest X-ray is negative. A lateral chest X-ray may assist in these cases (White et al. 1993).
Pneumothorax is a rare manifestation of lung cancer and may be associated with pleural effusion. It was estimated that the occurrence of air in the pleural space is less than 1 % in patients with lung cancer (Kabnick 1982; Steinhausling and Cuttat 1985; Woodring 1990). Also, lung cancer is the cause of only 0.05 % of pneumothorax cases (Steinhausling and Cuttat 1985). A pneumothorax may develop as a complication of a bronchopleural fistula secondary to both pleural and bronchial infiltration by the tumor, from a peripheral necrotizing tumor invading the pleura or from obstructive hyperinflation due to central obstruction (Steinhausling and Cuttat 1985; Woodring 1990). In most of the cases, pneumothorax occurs when lung cancer is already at an advanced stage (Woodring 1990).
The extension of the neoplasm to the pleura can also be assessed by chest computed tomography (Mountain 1997; Au and Thomas 2003). Chest computed tomography is sensitive in recognizing a pleural effusion, but cannot identify its possible malignant nature (McLoud 1998; Armstrong et al. 1995). However, some patterns may indicate malignancy, such as a pleural thickness over 1 cm indicating pleural carcinomatosis (Leung 1990). It is controversial whether computed tomography is more sensitive than the standard chest X-ray in recognizing associated findings such as a peripheral nodule and/or mass infiltrating the thoracic wall, the diaphragm, or the mediastinum (Armstrong et al. 1995; Glazer et al. 1989; Rato et al. 1991), as soft tissue swelling may be due to inflammation and/or fibrosis rather than direct infiltration of tumor (Pearlberg et al. 1987; Wang et al. 2004). Also, it appears that focal chest pain is more accurate than chest computed tomography in predicting chest wall invasion (Glazer et al. 1985). Computed tomography may also reveal mediastinal lymphadenopathy or associated pathology in the contralateral lung or pleura. The presence of these findings may be indicative of the malignant nature of the pleural effusion (Woodring 1990; Leung et al. 1990; Glazer et al. 1985).
Magnetic resonance imaging is less sensitive than computed tomography in diagnosing a pleural effusion (Au and Thomas 2003; McLoud 1998; Webb 1989). On T1-weighted images the signal from the fluid is very low and therefore may not be detected, although characteristic brightening on T2-weighted images allows detection (Au and Thomas 2003; Webb 1989; Templeton et al. 1990). In addition, magnetic resonance imaging has the same limitations as computed tomography in recognizing chest wall, mediastinal, pericardial, or diaphragmatic infiltration (Armstrong et al. 1995; Webb 1989; Templeton et al. 1990).
In malignant pleural disease, chest imaging with positron emission tomography (PET) using fluorine 18-labeled fluorodeoxyglucose (FDG) has a sensitivity ranging from 93 to 100 % and specificity from 67 to 89 %. The negative predictive value of the PET ranges from 94 to 100 % and its positive predictive value from 63 to 94 % (Duysinx et al. 2004; Erasmus et al. 2000; Schaffler et al. 2004). False-positive results occur in patients with any other inflammatory process such as parapneumonic effusions or pleurodesis after talc instillation (Wang et al. 2004; Kwek et al. 2004). When PF cytology is negative, a negative PET-FDG scan provides the most useful clinical information for ruling out a pleural effusion of malignant etiology (negative predictive value) (Wang et al. 2004).
Ultrasound is another noninvasive method for investigating the pleura (Herth 2004). Although its major indication is loculated pleural effusions usually due to infection, it may be also helpful in patients with a low performance status who are unsuitable for a more sophisticated examination, such as computed tomography of the thorax (O’Moore et al. 2004; Lipscomb et al. 1981). Ultrasound sensitivity in setting of pleural effusion is 92 % alone; combined with the standard chest radiograph, it is 98 % (Lipscomb et al. 1981; Henschke et al. 1989). It also assists in the recognition of associated findings such as pleural thickness, pleural or subpleural tumors, and parenchymal masses (Herth 2004; Yang et al. 1992).
9.5 Pleural Thoracentesis
The associated chemistry of pleural fluid in patients with malignancy has been the subject of many reports. There are no specific features of pleural fluid chemistry from patients with lung carcinoma when compared to those with extrapulmonary malignancy to the pleura (Sahn 1982; Sahn 1998). Thus, as in other malignant pleural effusions, we expect to find an exudate, with a protein concentration >3 g/dL levels (or pleural to serum protein ratio >0.5) and lactate dehydrogenase (LDH) >200 IU/L (or pleural to serum LDH ratio >0.6) (Light et al. 1972, 1973a).
Some pleural fluid findings are useful in confirming the diagnosis (Table 9.2). In approximately 30 % of cases, the pH will be less than 7.30 and glucose levels less than 0.6 g/dL (or the ratio of pleural to serum glucose <0.5) (Light 1995; Light et al. 1973b; Berger and Maher 1971; Chavalittamrong et al. 1979; Good et al. 1985). Low pleural fluid pH (<7.20) may indicate trapped lung, a shorter expected survival, and a higher probability of a failed pleurodesis (Sanchez-Armengol and Rodriguez-Panadero 1993; Sahn 1998; Rodriguez-Panadero and Antony 1997). However, Aelony et al. reported that thoracoscopic talc poudrage was successful in 88 % of patients with malignant pleural effusion despite the low pH (Aelony et al. 1998). In malignant pleural effusions, when both low pH and glucose are present, there is an association with marked pleural thickening and inhibition of transfer between the pleural space and the systemic circulation (Rodriguez-Panadero and Lopez Mejias 1989). In rare occasions the pleural fluid is a transudate due to associated diseases such as congestive heart failure, atelectasis from bronchial obstruction, or hypoalbuminemia (Chernow and Sahn 1977; Sahn 1982). Tests to define whether the fluid is an exudate or transudate are not diagnostic, but provide a probability as to the likely nature of a pleural effusion (Heffner 1998).
Table 9.2
Pleural fluid tests in patients with exudative pleural effusion
Test of pleural fluid | Test value | Probability |
|---|---|---|
pH | <7.30 | In 30 % of malignant PE |
Glucose | <0.6 g/dl | In 30 % of malignant PE |
Red cells | >100,000/ml | High probability for malignancy |
Lymphocytes | >50 % | Probable malignancy, do not rule out TB |
Adenosine deaminase (ADA) | >40 U/L | High probability for TB (used in countries with high TB incidence) |
Eosinophils | >10 % | Also present in malignancy |
Cytology | >0 | Malignant PE |
Cytology | <0 | Do not rule out malignancy |
PCR for TB | <0 | Rule out TB |
Aneuploidy | >0 | High probability for malignancy |
Leukocytes in malignant pleural fluid are relatively low with mean values ranging from 2,000 to 2,500 cells/mL (Sahn 1998; Chernow and Sahn 1977). While the total amount of leukocytes is not helpful in the differential diagnosis, the type of cell is important. In malignant pleural effusions we may find the proportion of lymphocytes to be greater than 50 % (Chernow and Sahn 1977; Domagala et al. 1978). Neutrophils usually represent less than 25 % (Sahn 1998), while total eosinophils are low (7–10 %) (Kuhn et al. 1989; Rubins and Rubins 1996). Other nucleated cells may be found, such as macrophages and mesothelial cells. Erythrocytes (Table 9.2) average about 40,000–50,000 cells/mL, however, with a wide range dependent on the etiology, from none to hemothorax (Chernow and Sahn 1977).
Several tumor markers, such as carcinoembryonic antigen (CEA), CA-125, CA 19-9, CYFRA 21-1, and NSE, have been tested in patients with malignant pleural effusions (Cascinu et al. 1997; Alatas et al. 2001). Although results seem to be contradictory as to the usefulness of these markers in the differential diagnosis of pleural effusions even when comparing patients with malignant and nonmalignant effusions, some authors propose specific tumor markers for the diagnosis of pleural effusions due to bronchogenic carcinoma (Menard et al. 1993). A reasonable compromise may be that tests should be performed in a selected population of patients with negative cytology and a “suspect” clinical outcome (Falcone et al. 1996). Recently, a number of reports have emerged, studying various novel markers, such as oncogenes (Stoetzer et al. 1999), various cytokines involved in inflammation (Alexandrakis et al. 2000), or matrix metalloproteinases (Hurewitz et al. 1992) as predictive or prognostic indicators of malignant pleural effusions. Until now, none of these markers have proved their usefulness even in differentiating malignant from benign pleural effusions. Generally, biochemical or biological markers in the malignant pleural effusions, as well as in the serum, cannot replace the routine cytopathological examination in the diagnosis of the disease (Marel et al. 1995).
A diagnosis of malignant pleural effusion can be made only when cancer cells are found in the pleural fluid. The first step in the assessment is cytological examination of the pleural fluid obtained at the time of thoracentesis (Sahn 1998; Antony et al. 2001). Cytologists face two major problems: to prove malignancy by confirming the presence of malignant cells and, in addition, to establish the organ of origin of those malignant cells. The diagnostic accuracy of the cytological examination of the pleural fluid varies from series to series. It is very low for some authors ranging from 15 to 35 % (Storey et al. 1976; Salyer 1975), while very high for others ranging from 80 to 90 % (Johnston 1985; Light et al. 1973a). This yield is increased only slightly (approximately 20 %) if repeated cytology specimens are sent (Johnston 1985). The cytological yield is higher for adenocarcinoma and when smears and blocks are used (Johnston 1985). Variations in diagnostic yield are due to various factors including the stage of the disease and the origin of the primary malignancy (Antony et al. 2001).
9.6 Minimally Invasive Techniques
Blind pleural biopsy have similar results to pleural cytology (Salyer et al. 1975). However, the combination of both techniques seems to improve the diagnostic yield (Johnston 1985; Antony et al. 2001). The low diagnostic yield of closed pleural biopsy is explained by a number of factors including minimal pleural extension; localization of tumors in regions of the pleura unreachable by biopsy needle, including the visceral pleura (Canto et al. 1983); and the level of experience of the physician (Walshe et al. 1992). Furthermore, the diagnostic yield of blind biopsy increases with the number of specimens taken in malignant pleural effusion (Jimenez et al. 2002). At least four biopsy samples are needed for accurate diagnosis (Jimenez et al. 2002). Since malignant pleural invasion is preferentially located at the bases of the hemithorax, it is recommended the sample be taken from the lowest region of the costal pleura in order to achieve a higher diagnostic success (Canto et al. 1983; Rodriguez-Panadero et al. 1989). However, Prakash reported that only 20 out of 281 patients (7 %) with malignant pleural effusion and initially negative fluid cytology had a closed biopsy which confirmed the disease (Prakash and Reiman 1985). Therefore, this result argues against performing concurrent thoracentesis and closed biopsy as an initial step, when a malignant origin for the pleural effusion is suspected. In cases with initial negative cytology and an indeterminate exudative pleural effusion, it may then be reasonable to repeat the thoracentesis with a pleural biopsy. However, negative pleural cytology and closed biopsy do not rule out a malignant origin for the pleural effusion. On the contrary, both techniques are indicated as the initial approach when considering a tuberculous pleural effusion as the diagnostic yield from the biopsy is consistently higher than that found in malignant disease (Prakash and Reiman 1985; Loddenkemper et al. 1978).
When pleural thoracentesis and/or biopsy does/do not show malignant cells, and lung carcinoma is highly suspected, it is reasonable to perform a fiber-optic bronchoscopy, since it may help in obtaining a diagnosis (Vergnon and Froudarakis 1999). Pleural effusions of unknown origin after the initial work-up are associated with bronchogenic carcinoma in about 30 % of the cases (Vergnon and Froudarakis 1999; Poe et al. 1994). In addition, fiber-optic bronchoscopy is important in assessing the extent of the disease in the tracheobronchial tree, which may be important for the patient’s treatment and prognosis (Vergnon and Froudarakis 1999).
9.7 Thoracoscopy in the Diagnosis of Malignant Pleural Effusion
Thoracoscopy is the “gold standard” in the diagnosis of pleural effusion, and it is indicated when less invasive tests have failed (Maskell and Butland 2003; Colt 1999; Mathur et al. 1995; Boutin and Astoul 1998). Thoracoscopy is a simple and safe method and should be the procedure of choice in the diagnosis of an exudative pleural effusion (Mathur et al. 1995; Boutin and Astoul 1998). This minimally invasive method can be performed by a pulmonologist under local anesthesia in the endoscopy suite, thus sparing the patient more invasive techniques such as video-assisted thoracic surgery (VATS) or open thoracotomy as performed by the thoracic surgeons under general anesthesia with selective ventilation in an operating theater (Mathur and Loddenkemper 1995). Before performing a thoracoscopy and to minimize complications, the performance status and expected benefit in terms of patient’s overall survival should be considered. Other relative contraindications such as coagulation disorders, with or without anticoagulant therapy; thrombocytopenia; severe respiratory insufficiency with hypercapnea; and unstable cardiac status must also be ruled out and if possible corrected prior to undertaking the thoracoscopy (Froudarakis 2008; Rodriguez-Panadero et al. 2006).
The sensitivity of thoracoscopy ranges from 92 to 97 % (Boutin et al. 1981, 1993a), and its specificity is 99–100 % (Boutin et al. 1981, 1993 b; Roeslin et al. 1992) for patients with a malignant pleural effusion (Table 9.3). The diagnostic sensitivity of thoracoscopy does not alter according to the origin of the malignant pleural effusion: it is 96 % for lung carcinomas, 92 % for mesotheliomas, and 96 % for extrathoracic metastatic malignancies (Antony et al. 2001). Biopsies may be taken not only from the parietal, but also from the visceral and diaphragmatic pleuras, since during thoracoscopy the whole pleural cavity is inspected under direct vision (Boutin et al. 1981; Loddenkemper 1998). In addition, there is the possibility to perform lysis of adhesions which may have limited the access to the pleural cavity (Antony et al. 2001; Rodriguez-Panadero et al. 2006). It appears that the recently developed semirigid (or semiflexible, or flex-rigid) thoracoscope has the same diagnostic accuracy as the classical rigid one (Ernst et al. 2002; Lee 2007). However, problems exist as biopsies are usually of a smaller size than those done with the rigid scope, as it utilizes a flexible forceps (same as flexible bronchoscopy) (Lee and Colt 2005a). This is especially an issue in patients suspected of malignant mesothelioma, where large biopsies are needed for accurate histological subtyping (Froudarakis 2008). The role of thoracoscopy with autofluorescence in undiagnosed pleural effusions is still under investigation. Primary results showed a high sensitivity (100 %) but low specificity (75 %) for malignant pleural effusion due to false-positive fluorescence of nonspecific pleuritis (Chrysanthidis and Janssen 2005).
Table 9.3
Diagnostic accuracy of thoracoscopy for malignant pleural effusion
Series | Patients (n) | Thoracoscopy (n) | Malignancy with thoracoscopy/total pleural malignancies (%) | True benign disease (n) | Idiopathic pleuritis (n) |
|---|---|---|---|---|---|
De Camp et al (1973) | 126 | 121 | 47/50 (94 %) | 32 | 44 |
Canto et al (1977) | 208 | 172 | 129/137 (94 %) | 12 | 59 |
Weissberg et al (1980) | 127 | 73 | 69/69 (100 %) | – | – |
Boutin et al (1980) | 233 | 195 | 131/150 (88 %) | 25 | 40 |
Boutin et al (1981) | 215 | 215 | 131/150 (87 %) | 25 | 40 |
Page et al (1989) | 121 | 107 | 90/91 (99 %) | 15 | 15 |
Wu et al (1989) | 152 | 152 | 71/74 (96 %) | 63 | 15 |
Hucker et al (1991) | 102 | 102 | 61/76 (80 %) | 5 | 21 |
Menzie et al (1991) | 102 | 91 | 42/44 (96 %) | 31 | 22 |
Ohri et al 1992 | 56 | 56 | 37/38 (97 %) | 11 | 7 |
Harris et al. (1995) | 182 | 124 | 70/73 (96 %) | 25 | 26 |
Colt (1995) | 52 | 28 | 12/12 (100 %) | 14 | 2 |
Hansen et al (1998) | 147 | 147 | 91/103 (88 %) | 11 | 33 |
Janssen et al (2004) | 709 | 709 | 318/349 (91 %) | 183 | 177 |
Ferrer et al (2005) | 93 | 93 | 50/56 (89 %) | 26 | 11 |
Sakuraba et al (2006) | 138 | 138 | 37/39 (95 %) | 40 | 58 |
Simpson (2007) | 89 | 89 | 73/77 (94.5 %) | – | – |
Fletcher et al (2007) | 50 | 50 | 40/42 (95 %) | 3 | 4 |
Lee et al (2007) (flex-rigid) | 51 | 51 | 34/36 (94 %) | 13 | 2 |
Macroscopically, the lesions seen during thoracoscopy may vary from polypoid tumors, masses, “candle wax drops” (Fig. 9.1), nodules, and/or pleural thickening – diffuse or localized, with associated islands of metastatic invasion from uninvolved pleura (Colt 1999; Mathur et al. 1995; Boutin and Astoul 1998; Rodriguez-Panadero et al. 2006). Also, nonspecific inflammation, localized or diffuse, may be observed, which has to be biopsied (Canto et al. 1983). Biopsies of the parietal pleura should be performed over a rib so as to avoid the neurovascular bundle. The closed forceps should be first used to probe the rib in order to feel the hard undersurface; this is followed by the grasping of the parietal pleura overlying it and removing the pleura with a long tearing motion (“peeling”), rather than a “grab and pull” (Lee and Colt 2007). Multiple biopsies have to be taken (>10) of the abnormal areas as well as additional “bites” of the same area (deep biopsy) to obtain tissue of sufficient depth for accurate diagnosis (Rodriguez-Panadero et al. 2006; Lee and Colt 2007).
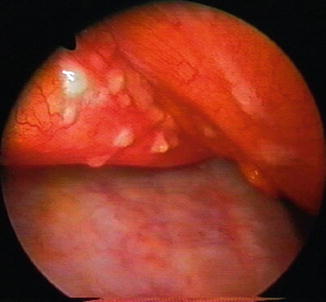

Fig. 9.1
Candle wax metastatic nodules on the pericardium of a 70-year-old patient with a pleural effusion from breast adenocarcinoma. Note the diffuse invasion of the parietal pleura
Survival of patients with malignant pleural effusion is very much related to the primary tumor, with ovarian tumors having the longest survival and lung tumors the shortest (Lee and Colt 2007; Froudarakis 2009). The biopsies taken must be of significant number and size in order to identify tumor invading the pleura and to perform additional investigations such as hormonal receptors in the case of breast carcinoma (Schwarz et al. 2004). Lymphoma is also difficult to diagnose by thoracentesis and closed biopsy, and thoracoscopy should be performed if this tumor is suspected, and initial investigations are negative (Alifano et al. 1997; Celikoglu et al. 1992). Lymphomatous pleural effusion usually results from impaired lymphatic drainage of the pleural space as well as from direct pleural involvement (Steiropoulos et al. 2009). Large and numerous biopsy specimens are necessary for immunohistochemistry/molecular techniques to identify and subclassify lymphomas (Fig. 9.2) (Celikoglu et al. 1992; Alifano et al. 1997; Steiropoulos et al. 2009).
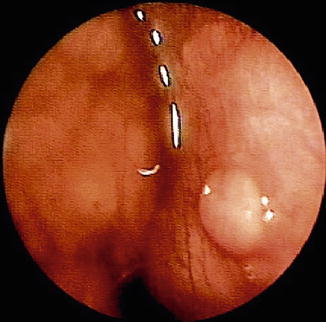

Fig. 9.2
Invasion of the parietal pleura from Hodgkin’s lymphoma as discovered during thoracoscopy in a 39-year-old patient with a pleural effusion. In addition to the nodule, there is diffuse invasion of the parietal pleura as well as a mass within the mediastinum
Some authors have attempted to identify features which would give a high pretest probability of malignancy. Martensson performed logistic analysis using seven variables (the patient’s sex, age, smoking habits, asbestos exposure, size of effusion, pleural fluid color, and eosinophils) to discriminate malignant from nonmalignant disease in 334 patients with undiagnosed pleural effusion, in order to better select patients for diagnostic thoracoscopy (Martensson 1989). His predictions were correct in 79 % of the effusions; the strongest variable for malignancy was the presence of a bloody effusion. On the contrary, the variables with the strongest negative predictive value for malignancy were the presence of eosinophils (>30 %) in the pleural fluid as well as patient age younger than 50 years (Martensson 1989). Harris has published a series of 182 patients with undiagnosed pleural effusion who underwent thoracoscopy for diagnosis. He observed that a previous history of malignancy and an age older than 50 years were statistically significant as positive predictors for identifying malignancy by thoracoscopy. The combination of high pleural LDH, lymphocytosis, and hemorrhagic fluid was associated with intrapleural malignancy, and therefore, patients with those characteristics should undergo diagnostic thoracoscopy. In the same study, patient management was directly affected by thoracoscopy in 85 % of patients (Harris et al. 1995).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


