Cardiac involvement in systemic amyloidosis causes detrimental prognosis; therefore, early detection and classification are important to develop appropriate therapeutic strategies. Subcutaneous tissue biopsy is a useful screening procedure for systemic amyloidosis; however, its diagnostic and prognostic value in patients with cardiac amyloidosis remains elusive. Thus, we investigated the value of subcutaneous tissue biopsy in cardiac amyloidosis. In 22 patients with cardiac amyloidosis, we retrospectively analyzed 14 consecutive patients with cardiac amyloidosis who underwent subcutaneous tissue biopsy. Amyloid deposition was observed in 11 patients (79%). Histopathologic analysis demonstrated that acquired monoclonal immunoglobulin light-chain amyloidosis could be predicted when the degree of amyloid deposition was greater in blood vessels than adipose tissue compared to senile systemic amyloidosis and familial amyloidosis (60% vs 0%, p = 0.03). During the follow-up period (median 297 days, range 3 to 761), 7 patients (5 with monoclonal immunoglobulin light-chain amyloidosis and 2 with senile systemic amyloidosis) died or were admitted to the hospital because of worsening heart failure. Of them, 6 patients (86%) were positive for amyloid deposition in blood vessels in subcutaneous tissue biopsy. Incidence of death and composite outcome including heart failure hospitalization and death was significantly higher in patients positive for amyloid deposition in blood vessels than in those without (p = 0.03, p = 0.006, respectively). These results suggest that amyloid subtype could be diagnosed by assessing the degree of amyloid deposition in blood vessels and adipose tissue in subcutaneous tissue biopsy samples from patients with cardiac amyloidosis. Amyloid deposition in blood vessels suggests poor prognosis of these patients.
The precise diagnosis of cardiac amyloidosis requires endomyocardial biopsy to demonstrate amyloid deposition, but this procedure is relatively invasive and cannot be performed routinely. Recent developments in the diagnostic accuracy of late gadolinium-enhancement cardiac magnetic resonance and sampling of another tissue have minimized the need for diagnostic invasive endomyocardial biopsy. For example, aspiration of abdominal fat pad by fine-needle aspiration is a safe and reliable screening procedure. Subcutaneous tissue biopsy is also recognized as an informative screening technique for patients with amyloidosis ; however, little information is available on its diagnostic and prognostic value. In the present study, we investigated the value of such biopsy in patients with cardiac amyloidosis.
Methods
Twenty-two patients were diagnosed with cardiac amyloidosis from August 2007 through May 2012 at Kumamoto University Hospital. Data of 14 consecutive patients who underwent subcutaneous tissue biopsy were available for retrospective analysis. Clinical outcome and cause of death were retrieved from medical records until May 2012. The study protocol was approved by the human ethics review committee of Kumamoto University and a signed consent form was obtained from each subject.
Diagnosis of systemic amyloidosis was based on Congo red staining and apple-green birefringence examination under cross-polarized light in ≥1 involved organ such as abdominal subcutaneous adipose tissue, gastrointestinal tract, or heart. Cardiac amyloidosis was diagnosed by amyloid disposition in the myocardium or increased wall thickness on echocardiogram in the absence of any other cause of ventricular hypertrophy provided a histopathologic diagnosis of amyloidosis was made from another tissue. A positive cardiac histologic result for cardiac amyloidosis was available in 5 patients by endomyocardial biopsy or autopsy examination. Presence of global transmural or subendocardial late gadolinium-enhancement cardiac magnetic resonance strongly supported the diagnosis of cardiac amyloidosis in 5 patients. The other 4 patients were diagnosed by echocardiographic findings and histopathologic diagnosis of amyloidosis.
Immunohistochemistry and DNA analysis were employed to determinate the subtype classification of cardiac amyloidosis. Acquired monoclonal immunoglobulin light-chain (AL) amyloidosis was confirmed by evidence of monoclonal protein in serum or urine and/or a monoclonal population of plasma cells in bone marrow. Senile systemic amyloidosis (SSA) was diagnosed by positive immunohistochemistry findings for transthyretin in the absence of any transthyretin-related mutation at DNA analysis with negative findings of AL amyloidosis. Familial amyloidosis was diagnosed by documented transthyretin-related mutation at DNA analysis.
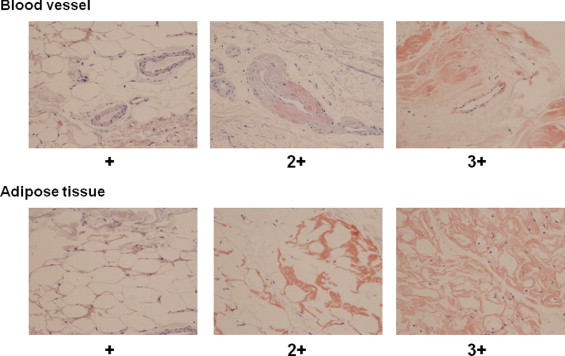
Subcutaneous tissue evaluation was performed as follows. After disinfection and local anesthesia with 1% lidocaine (Xylocaine, AstraZeneca, Osaka, Japan) injected intradermally using a small needle, a fingertip-size piece of skin that included the epidermis, dermis, and sufficient amount of subcutaneous adipose tissue was obtained from the abdominal wall with a number 15 scalpel. Immediately after removal, samples were fixed in formalin and then embedded in paraffin. For classification of amyloid protein, wax-embedded sections were pretreated with or without potassium permanganate (KMnO 4 ) and then stained with Congo red. Non-amyloid A protein was identified by positive staining with Congo red and insensitivity to KMnO 4 , whereas amyloid A protein was sensitive to KMnO 4 treatment. Deposition of amyloid on adipose tissues and/or blood vessel walls was examined under a light microscope (model BX50, Olympus, Tokyo, Japan). Congo red–stained slides were scored semiquantitatively under the light microscope using a scale of − to +3 as shown in Figure 1 . Slides were reviewed by 2 independent observers who were unaware of the clinical condition at 400× magnification with 5 smears per patient. Scales >+1 were considered significant positive staining.
Skewed data were presented as median with interquartile range. Differences between groups were examined by Mann–Whitney U test for unpaired data. Categorical values were compared by Fisher’s exact test. Kaplan–Meier survival analysis was used to evaluate the association between findings of subcutaneous tissue biopsy and survival with log-rank test used to determine probability value. A 2-tailed p value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS 19 (SPSS, Inc., Chicago, Illinois).
Results
Table 1 lists the clinical characteristics of 14 patients with cardiac amyloidosis. Eleven of 14 patients were positive for amyloid fibril deposition in subcutaneous tissue biopsy. Sensitivity of subcutaneous tissue biopsy for diagnosis of cardiac amyloidosis was 79%. Amyloid deposition was not detected in subcutaneous tissue biopsy of 3 patients (cases 3, 4, and 10) who were otherwise diagnosed with amyloidosis by identifying amyloid deposition in the heart (cases 3 and 4) and duodenum (case 10). No patient developed complications related to subcutaneous tissue biopsy.
| Case | Age (years)/Sex | Amyloid Type | NYHA | BNP (pg/ml) | VST (mm) | LVEF (%) | LAD (mm) | Peak E (cm/s) | Peak A (cm/s) | E/A | E/e′ | Blood Vessels | Adipose Tissue | Follow-Up (days) | Event |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70/F | AL | III | 1,874 | 19 | 54 | 52 | 71 | 24 | 2.9 | 26.6 | 2+ | + | 457 | Death |
| 2 | 78/F | AL | III | 599 | 17 | 45 | 41 | 87 | 78 | 1.1 | 31.0 | + | + | 289 | Death |
| 3 | 56/M | SSA | III | 427 | 21 | 60 | 51 | 85 | 43 | 2.0 | 15.1 | — | — | 761 | NE |
| 4 | 76/M | SSA | II | 92 | 12 | 56 | 40 | 51 | 78 | 0.7 | 7.7 | — | — | 604 | NE |
| 5 | 61/M | AL | IV | 1,300 | 14 | 52 | 41 | 101 | 52 | 1.9 | 18.1 | — | 2+ | 56 | Death |
| 6 | 85/M | SSA | II | 181 | 14 | 55 | 52 | 66 | 41 | 1.6 | 8.0 | — | + | 625 | NE |
| 7 | 65/M | AL | IV | 2,481 | 22 | 60 | 32 | 82 | 66 | 1.2 | 28.5 | 2+ | + | 37 | Death |
| 8 | 79/M | AL | IV | 2,387 | 14 | 31 | 42 | 79 | 19 | 4.0 | 30.3 | 2+ | + | 59 | Death |
| 9 | 70/M | SSA | III | 441 | 22 | 43 | 40 | 45 | 52 | 0.9 | 23.6 | 2+ | 2+ | 543 | HF |
| 10 | 83/M | SSA | I | 329 | 18 | 46 | 36 | 80 | 27 | 2.9 | 22.0 | — | — | 543 | NE |
| 11 | 80/M | SSA | II | 125 | 19 | 45 | 31 | 45 | 62 | 0.7 | 8.7 | — | 2+ | 305 | NE |
| 12 | 78/F | SSA | IV | 499 | 9 | 49 | 41 | 114 | 51 | 2.2 | 44.2 | 3+ | 3+ | 18 | Death |
| 13 | 70/M | SSA | III | 157 | 14 | 62 | 35 | 59 | 49 | 1.8 | 21.0 | — | + | 242 | NE |
| 14 | 59/M | familial | III | 269 | 25 | 40 | 49 | 79 | 20 | 4.0 | 20.3 | — | 2+ | 3 | NE |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


