Diagnosis and Treatment of Pulmonary Embolism
Jeffrey V. Garrett
Thomas C. Naslund
Pulmonary embolism (PE) has long been recognized as a major health care concern. Historical studies have cited an untreated mortality rate of approximately 30%; however, study design variables have made these results difficult to interpret. Although the incidence of clinically significant PE has been estimated to be 600,000 cases per year, diagnostic inaccuracy and variable clinical presentations prevent calculation of the true incidence of PE in the general population.
Inarguably, clinically significant PE contributes to substantial patient morbidity and mortality each year. Objective testing is crucial, because clinical assessment or simple laboratory tests are unreliable and the consequences of misdiagnosis are serious. An incorrect diagnosis of PE unnecessarily exposes patients to the risks of treatment, and failure to make the diagnosis is associated with risk of mortality.
Pulmonary angiography has been long considered the diagnostic gold standard, with an accuracy of 90%. However, angiography is invasive and costly. To overcome these limitations, ventilation/perfusion V/Q scanning was introduced as a noninvasive alternative. Although it was initially considered accurate, many studies have demonstrated the limitations of this modality. The Prospective Investigation of Pulmonary Embolism Diagnosis Study (PIOPED) in 1990 defined a method for determining the presence or absence of PE with reasonable certainty in 96% of patients. However, in clinical settings, the use of the PIOPED approach is uncommon. Because of inconsistency in clinical evaluation, PE continues to be both an underdiagnosed and overdiagnosed disease. This chapter will review the current methodology in diagnosing and treating PE.
Clinical Features
The clinical features of PE are frequently variable, vague, and nonspecific. The diagnosis should be considered in any patient who presents with acute dyspnea, chest pain, syncope, or shock. Common symptoms in decreasing order of frequency include dyspnea, pleuritic chest pain, anxiety, cough, hemoptysis, sweats, nonpleuritic chest pain, and syncope. Common signs in decreasing order of frequency include tachypnea (respiratory rate >16), tachycardia (heart rate >100), fever, phlebitis, cardiac gallop, diaphoresis, edema, and cyanosis. Massive PE presents with hypotension and hypoxia. A past history of deep venous thrombosis (DVT) has been reported to exist in 30% of cases. More than 90% of PE arise from lower-extremity DVT, although the clinical signs of DVT are apparent in only 10% of cases. Abdominal symptoms are notably infrequent.
Diagnostic Considerations
The initial workup of a suspected PE should include arterial blood gas analysis, electrocardiography (ECG), and chest radiograph. These tests are unreliable in diagnosing PE but may provide important information that excludes or supports an alternative diagnosis. Investigations specifically aimed at diagnosing PE include V/Q lung scanning, pulmonary angiography, echocardiography, and spiral computed tomography (CT).
Arterial Blood Gases
In the absence of cardiopulmonary disease, 38% of patients in the PIOPED study with PE had a normal blood gas analysis. For those patients with pre-existing disease, 14% with documented PE had normal blood gases. Although of limited value, typical blood gas abnormalities include hypoxia, hypocarbia, and an elevated alveolararterial oxygen gradient.
Electrocardiography
A completely normal ECG is seen in less than 10% of patients with documented PE. However, the classic S1Q3T3 on the ECG is present in only 12% of cases. T wave inversion in one or more of the precordial leads is frequently cited as the most common ECG finding. Reversibility of this sign with thrombolyis has been shown to predict a good clinical outcome.
Chest Radiography
Chest radiography has poor sensitivity and specificity in the diagnosis of PE; thus, when taken alone, it is of limited value. However, a plain chest radiograph is an essential part of the initial diagnostic investigation, as it may exclude alternative pathology. In the PIOPED study, 12% of patients with PE had normal chest radiographs. The most common finding was atelectasis, but this was not specific to PE. Further radiographic signs that may be seen include pleural effusion, pulmonary artery prominence, cardiomegaly, elevated hemidiaphragm, pulmonary infarction, and an enlarged hilum.
D-dimer Blood Testing
D-dimer is formed when cross-linked fibrin is lysed by plasmin. Elevated levels are expected to occur with PE. The value of D-dimer is that a negative result can help to
exclude PE. However, the finding of an elevated D-dimer in hospitalized patients is nonspecific. More recently, the enzyme-linked immunosorbent assay (ELISA) D-dimer has been shown to have a sensitivity of 98% for venous thromboembolism, but problems with specificity, high frequency of false positives, and slow turnaround time limit its clinical utility.
exclude PE. However, the finding of an elevated D-dimer in hospitalized patients is nonspecific. More recently, the enzyme-linked immunosorbent assay (ELISA) D-dimer has been shown to have a sensitivity of 98% for venous thromboembolism, but problems with specificity, high frequency of false positives, and slow turnaround time limit its clinical utility.
Evaluation of Lower-extremity Veins
The majority of PE originate from DVT of the lower extremities. However, the possibility of PE cannot be ruled out on the basis of a negative lower-extremity ultrasound study. One has no assurance that some DVT remains in the lower extremity or all embolized to the lung. Furthermore, a positive study should be interpreted with caution, as it may represent findings of chronic DVT. Ultrasound may be most valuable in the face of suspicion of PE in a patient with extremity findings compatible with DVT. A positive study in this population would warrant treatment without further workup. A negative study would require further testing.
Ventilation/Perfusion Lung Scanning
The PIOPED study was a multicenter prospective study that compared pulmonary angiography with V/Q scanning. Using the angiogram as the gold standard, PIOPED found that 87% of patients with high probability V/Q scans had PE. Patients with intermediate-probability, low-probability, or normal scans had 30%, 14%, and 4% incidence of PE, respectively. Diagnostic accuracy was improved slightly when V/Q scans were combined with pretest clinical probability estimates of the physician. However, 33% of patients with intermediate-probability scan results and 16% with low-probability scan results had angiographically documented PE. Furthermore, patients with prolonged immobilization, lower-extremity trauma, recent surgery, or central venous instrumentation with low-probability scans were found to have a fourfold increased risk of PE when compared with patients without these risk factors. From these findings it is apparent that scans with the most clinical value are those that have a very low, low, or high probability of PE in patients who demonstrate a compatible clinical picture. According to the PIOPED analysis, most patients require pulmonary arteriography for definitive diagnosis.
More recent reviews have shown that a normal V/Q scan generally excludes PE, but it is only found in approximately 25% of patients. The likelihood that perfusion defects are due to PE increases with increasing number and size, the presence of a wedge shape, and the presence of a normal ventilation scan (mismatched defect). High-probability defects are those with mismatched perfusion defects that are segmental or larger. A single mismatch defect correlates with a PE prevalence of 80%. Three or more defects increase the prevalence to greater than 90%. However, 65% of patients with PE have intermediate- or low-probability lung scans and require further testing.
Computed Tomography Angiography
CT pulmonary angiography has increasingly become the modality of choice in the diagnosis of PE (Fig. 69-1). Unlike V/Q scanning and pulmonary angiography, it allows for direct visualization of emboli, as well as lung parenchymal abnormalities that may support the diagnosis of PE or provide an alternative diagnosis. In addition, the presence of pre-existing lung disease (a pitfall with V/Q scanning) has not been shown to influence the negative predictive value of CT angiography. Earlier studies demonstrated that CT was comparable to pulmonary angiography in the diagnosis of large emboli in segmental or larger arteries (Fig. 69-1). However, visualization of subsegmental/peripheral arteries was limited; thus, a negative CT scan did not “rule out” PE. Further disadvantages include exposure to radiation and contrast, as well as limited visualization secondary to motion artifact. Transportation to and monitoring during CT scanning are also issues in some critically ill patients.
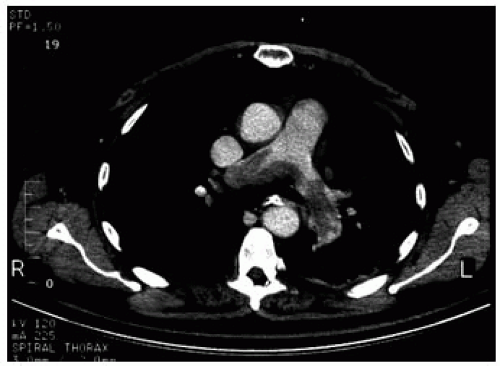 Figure 69-1. CT scan depicting central pulmonary embolus. (Adapted and reprinted with permission from Wells et al. Thromb Haemost. 2000;83:416-420.) |
Recent advances in CT technology have greatly improved the sensitivity of CT for subsegmental or peripheral emboli. Thin cut, multidetector spiral CT has been shown to have a sensitivity of 96% and a specificity of 98% in the detection of acute PE. The most important development with these high-quality scanners is the depiction of small peripheral emboli. Although occurring in 6% to 30% patients, the clinical implications remain controversial. However, the presence of peripheral emboli may be an indicator of concurrent DVT and warrant therapy to prevent a more severe embolic event.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


