3 Diabetes
 Introduction
Introduction
The Burden of the Disease
According to the American Diabetes Association (ADA), in 2007 diabetes affected 23.6 million individuals in the United States, corresponding to 11% and 21% of the population over 20 and 60 years of age, respectively.1 In the same year, 1.6 million new cases of diabetes were diagnosed. While 50% of these individuals were women, the condition remained unrecognized in 25% of those affected. In addition, the U.S. Department of Health and Human Services estimated that in 2004, approximately 40% of American adults aged 40 to 74 years, or 41 million, had prediabetes, a disturbance of glucose metabolism predisposing to overt diabetes, heart disease, and stroke.2 Also in the United States, the prevalence of diabetes is expected to more than double from 2005 to 2050.3 The proportional rises are projected to be largest in the elderly, with an expected increase of 220% among those 65 to 74 years of age and 450% ≥75 years of age, respectively. With respect to race, the most affected will be Hispanics, followed by African Americans. As a result, the prevalence of diabetes among African Americans >75 years of age is expected to increase by as much as 600%.3 One report has estimated that in the year 2010, the worldwide prevalence of diabetes among adults stood at 6.4% (285 million individuals), while it is expected to increase to 7.7% (439 million individuals) by the year 2030.4 The total estimated cost of diabetes in the United States in 2007 was $172 billion, divided into $116 billion for direct medical costs and $58 billion for indirect medical costs (e.g., disability, work loss).1 The total healthcare cost associated with this condition is expected to rise to $192 billion by the year 2020.5 After adjusting for age and gender, the average medical expenditures for individuals with diabetes are more than double those for their nondiabetic counterparts.
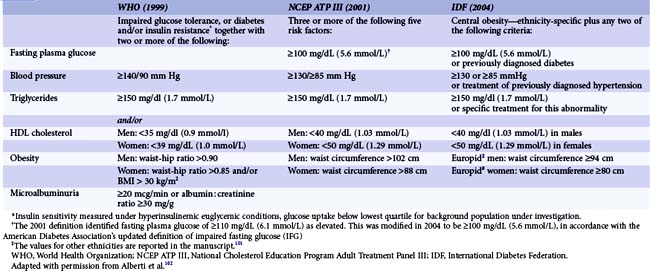
 Diagnostic Criteria of Diabetes, Prediabetes, and Metabolic Syndrome
Diagnostic Criteria of Diabetes, Prediabetes, and Metabolic Syndrome
The diagnostic criteria of diabetes according to the ADA are reported in Table 3-1. In addition to the long-standing established diagnostic criteria based on fasting plasma glucose or 75-g oral glucose tolerance test, in 2009 an International Expert Committee recommended the assessment of the hemoglobin A1C (HbA1c) to diagnose diabetes, with a threshold of >6.5%.6 Disturbances of the glucose metabolism, characterized by impaired fasting glucose levels or impaired glucose tolerance, can be detected long before the development of overt diabetes. These two metabolic abnormalities do negatively affect the CV system and were recently grouped under the term prediabetes (Table 3-1). Metabolic syndrome comprises a cluster of lipid and nonlipid risk factors of metabolic origin mediated by insulin resistance, such as pathological glucose metabolism, obesity, hypertension, and dyslipidemia. Several organizations have proposed definitions of the metabolic syndrome that may differ not only in the set of criteria included but also in the cut-offs to define the presence or absence of an individual component of the syndrome (Table 3-2). However, both the concept and the clinical utility of the metabolic syndrome have been critically appraised. Accordingly, a case-control study on the incidence of myocardial infarction (MI) performed in 52 countries and involving a total of 26,903 subjects showed that the risk of MI associated with metabolic syndrome was not greater than the sum of the risks associated with the components of this condition.7
TABLE 3-1 Diagnosis of Diabetes Mellitus, Impaired Glucose Tolerance, and Impaired Fasting Glucose According to the ADA
In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥200 mg/dL (11.1 mmol/L). |
ADA = American Diabetes Association.
* The test should be performed in a certified laboratory.
Reprinted with permission from the ADA.101
 Pathophysiology of Atherosclerosis in Diabetes
Pathophysiology of Atherosclerosis in Diabetes
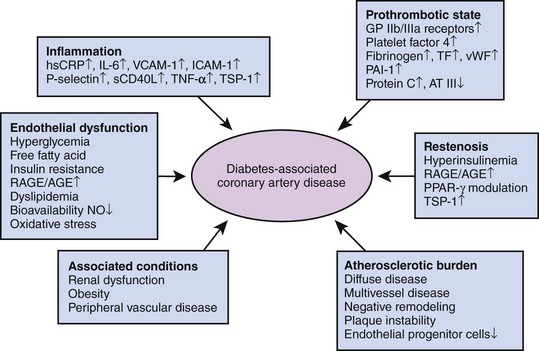
In patients with diabetes, CAD is more prevalent, more advanced, and occurs at a younger age compared with nondiabetic counterparts. Several metabolic abnormalities—including chronic hyperglycemia, dyslipidemia, oxidative stress, and insulin resistance—have been associated with the accelerated atherogenesis observed in diabetes (Fig. 3-1).8 In addition to metabolic disturbances, diabetes alters the function of multiple cell lines, including endothelial cells, smooth muscle cells, and platelets. Despite the description of several peculiarities characterizing diabetes-associated atherosclerosis, the exact mechanisms underlying the initiation and progression of the atherosclerotic process remain elusive.9
Prothrombotic State
The observation that diabetic patients are characterized by a hypercoagulable state is based on both clinical and laboratory findings. The prothrombotic state seen in diabetes is related to endothelial dysfunction, impaired fibrinolysis, increased levels of coagulation factors, and enhanced platelet reactivity. Manifestations of atherothrombosis include sudden cardiac death, ACS, ischemic stroke, peripheral arterial ischemia (i.e., intermittent claudication and critical limb ischemia), and coronary stent thrombosis. An angioscopic study performed in ACS patients revealed that plaque ulceration and intracoronary thrombus were more frequent among diabetic patients than in nondiabetic individuals. Similarly, the incidence of thrombus was found to be higher in atherectomy specimens from patients with diabetes undergoing PCI than in those from nondiabetic patients. With respect to laboratory findings, subjects at various stages of diabetes proved to have increased numbers of activated platelets compared with healthy controls. Accordingly, platelets of diabetic individuals have a greater platelet activation and aggregation response to shear stress and platelet-activating agonists. In addition, an increased platelet-surface expression of the glycoprotein (GP) Ib receptor, which mediates binding to von Willebrand factor, and of the GP IIb/IIIa receptor, which mediates platelet-fibrin interaction, has been described. Finally, basal thromboxane B(2) is significantly increased in resting platelets from diabetic patients even in the absence of vascular complications and in cases of well-controlled diabetes. Moreover, both decreased endothelial production of the antiaggregants NO—as previously mentioned—and prostacyclin; increased levels of procoagulant agents such as fibrinogen, tissue factor, von Willebrand factor, platelet factor 4, and factor VII; and decreased concentrations of endogenous anticoagulants such as protein C and antithrombin III have been documented (Fig. 3-1). Finally, elevated levels of plasminogen activator inhibitor-1 (PAI-1) may impair endogenous tissue plasminogen activator-mediated fibrinolysis. Overall, diabetes is characterized by increased intrinsic platelet activation, decreased endogenous inhibition of platelet activity, and increased blood coagulation in the presence of impaired endogenous fibrinolysis.
Inflammatory State
Inflammation has been related not only to acute CV events but also to the initiation and progression of atherosclerosis. Several CV risk factors, including diabetes, may trigger an inflammatory state. Although it is plausible that metabolic disturbances associated with diabetes trigger vascular inflammation, the converse may also be true. Accordingly, C-reactive protein (CRP), a key proinflammatory cytokine in patients with atherosclerosis, has been shown to independently predict the risk of developing type 2 diabetes. Inflammatory parameters are elevated in diabetes; in the context of insulin resistance in the absence of overt diabetes, these include high-sensitivity CRP, Il-6, tumor necrosis factor (TNF)-α, and a circulating/soluble form of CD40 ligand (sCD40L) (Fig. 3-1). In addition, an increased expression of adhesion molecules such as endothelial (E)-selectin VCAM-1 and ICAM-1 has been detected. The morphological substrate of increased vascular inflammatory activity can be derived by an analysis of coronary atherectomy specimen of ACS patients, showing that tissue from diabetic patients exhibited a larger content of lipid-rich atheromas and a more pronounced macrophage infiltration compared with specimens from nondiabetic individuals. The receptor for AGE (RAGE) may play an important role in inflammatory processes and endothelial activation, likely accelerating the processes of coronary atherosclerotic development, especially in diabetic patients. It has been demonstrated that CRP upregulates RAGE expression in endothelial cells. These observations reinforce the mechanistic link in diabetes among inflammation, endothelial dysfunction, atherothrombosis, and, as detailed below, accelerated restenosis.
 Cardiovascular Disease in Diabetes
Cardiovascular Disease in Diabetes
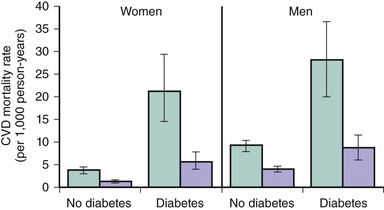
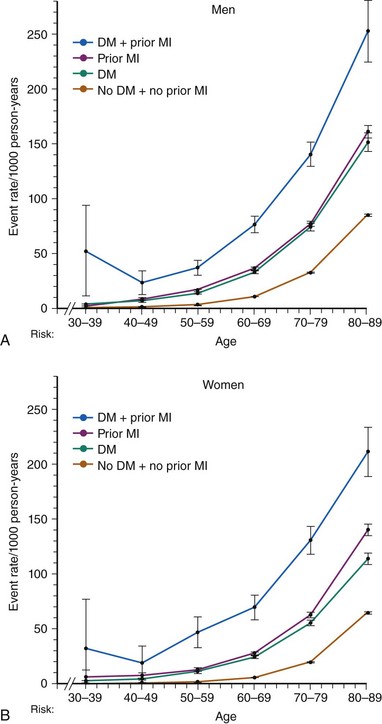
Adults with diabetes have a CV death rate that is up to fourfold greater than that of nondiabetic individuals. Moreover, among diabetic individuals, CVD accounts for over two-thirds of total mortality.2 With respect to gender, the adjusted risk of CV death among diabetic men is three times greater than that among their nondiabetic peers. Among diabetic women, the risk may be as high as sixfold as great as it is among those who are not diabetic. A population-based study has estimated that diabetes confers an equivalent CV risk to aging 15 years.10 Although in the western countries, such as the United States, the age-adjusted mortality rates of other major multifactorial diseases such as CAD, stroke, or cancer have declined or remained stable over the last 20 years, the diabetes “epidemic” has led to a 30% increase of diabetes-related deaths in the same time span.11 Nevertheless, the Framingham Heart Study documented a major reduction in CV mortality in both diabetic (hazard ratio [HR] 0.31) and nondiabetic (HR 0.38) individuals enrolled in the years 1975 to 2001 compared with those enrolled in the years 1950 to 1975 (Fig. 3-2).12 The absolute benefit was dramatic in the diabetic population, with mortality going from 24.1 to 6.8 per 1,000 person-years. Among nondiabetic individuals, it went from 6.3 to 2.4 per 1,000 person-years. Although mortality rates from CAD have declined in the western world during the past 30 years and people with diabetes have also benefited from this decline, the more than twofold higher risk of dying from CAD in men and women with diabetes has persisted over time.13 In 2001, the Adult Treatment Panel III of the National Cholesterol Education Program (NCEP ATP III) recommended considering diabetes as a CAD risk equivalent, therefore mandating aggressive CV prevention.14 This notion has been confirmed by a study including all residents in Denmark at least 30 years of age and following them for 5 years by individual-level linkage of nationwide registers. At baseline, 71,801 (2.2%) had diabetes mellitus and 79,575 (2.4%) had a prior MI. Regardless of age, the age-adjusted Cox proportional HR for cardiovascular death was 2.4 both in men with diabetes mellitus without a prior MI and in nondiabetic men with a prior MI, with nondiabetics without prior MI as the reference (Fig. 3-3).15
Peripheral Arterial and Cerebrovascular Disease
Epidemiological evidence confirms an association between diabetes and peripheral artery disease, with an incidence estimated to range between twofold and more than fourfold compared with nondiabetic individuals. In the Framingham cohort, the presence of diabetes increased the risk of claudication fourfold in men and ninefold in women. A study addressing the prevalence of peripheral artery disease among patients according to the degree of associated metabolic disturbance found that the rate of abnormal ankle-brachial indices ranged between 7% in individuals with normal glucose tolerance and 21% in those requiring multiple antidiabetic medications. Diabetes-associated vascular disease of the lower extremities is characterized by extensive vascular calcification and a more frequent infrapopliteal involvement. The lower limb amputation rate among diabetic patients is up to 13-fold compared with that among nondiabetic individuals. In 2004, over 71,000 lower limb amputations were performed in U.S. patients with diabetes, corresponding to over 60% of all nontraumatic lower limb amputations.16 Similar to what has been observed in the coronary and peripheral arterial circulation, diabetes triggers cerebrovascular disease. Accordingly, patients with diabetes more commonly have more advanced extracranial and intracranial atherosclerosis than do nondiabetic individuals. Case-control stroke studies and prospective epidemiological data have correlated poor glycemic control and stroke risk and have identified diabetes as an independent predictor of ischemic stroke, with an increased risk ranging from 1.8-fold to nearly 6-fold.2 Particularly ominous is the impact of diabetes in individuals below 55 years of age, with a greater than 10-fold increased risk of stroke. Finally, diabetes increases the risk of stroke-related dementia more than threefold, doubles the risk of recurrence, and increases total and stroke-related mortality.
 Cardiovascular Diagnostic Modalities in Diabetic Patients
Cardiovascular Diagnostic Modalities in Diabetic Patients
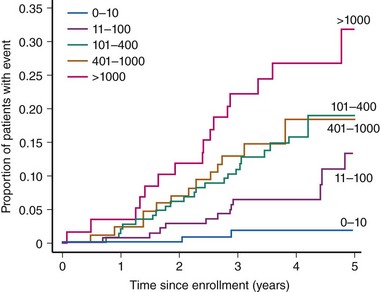
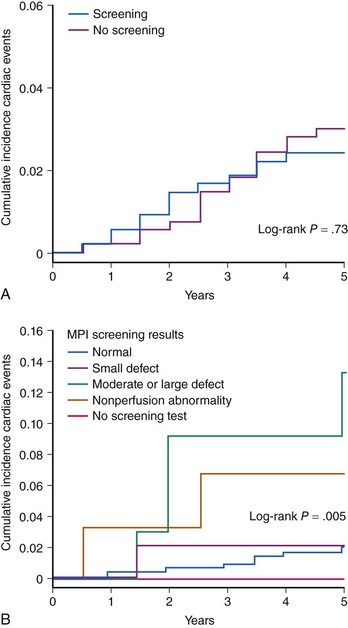
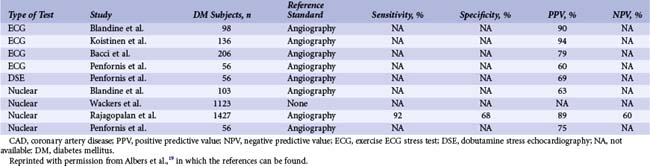
Diabetic patients have significantly higher rates of silent ischemia than the general population. It has been estimated that in the United States, as many as 12.5 million diabetic patients may have asymptomatic CAD.17 In the absence of typical symptoms, diabetic patients may suffer from myocardial ischemia more frequently than their nondiabetic counterparts, and most studies investigating the prevalence of silent ischemia have found higher rates in diabetic patients. The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study followed 9,795 diabetic patients aged 50 to 75 years with routine ECG for a mean of 5 years.18 In this study, 37% of all MIs identified were silent. Older age, longer diabetes duration, prior CVD, higher HbA1c levels, and albuminuria independently predicted the risk of silent MI. The diagnostic and prognostic value of stress testing diabetic patients has been extensively investigated (Tables 3-3 and 3-4).19 Exercise ECG testing is a well-established and inexpensive test to guide the clinician in the diagnosis and risk stratification of diabetic patients with suspected CAD. Sensitivity and specificity for the diagnosis of CAD in diabetic patients presenting with angina and in nondiabetic patients appear comparable. In asymptomatic patients, an abnormal exercise ECG test may be helpful to identify a subgroup of patients with advanced CAD. Patients with a negative stress test in the presence of normal exercise capacity are at low risk of CV events, at least in the short run. Stress nuclear imaging has the most extensive literature among the noninvasive imaging modalities for both diagnostic and prognostic purposes in diabetes. With respect to stress echocardiography, several studies have addressed its prognostic accuracy in diabetes, while the data on its diagnostic value are scarce (Tables 3-3 and 3-4).19 The assessment of coronary artery calcium is a well-established index of atherosclerosis. Electron beam computed tomography (CT) and multidetector CT make it possible to measure the calcium content of coronary arteries, and a scoring system has been developed to that purpose. Several studies have identified the coronary artery calcium score as a strong predictor for CV events and all-cause mortality in diabetic individuals. The PREDICT (Prospective Evaluation of Diabetic Ischaemic Disease by Computed Tomography) study evaluated prospectively calcium score as a predictor of CV events in 589 asymptomatic individuals with type 2 diabetes.20 The risk of a CV event rose with an increased calcium score (Fig. 3-4). In addition, calcium score had greater predictive value for events than a broad range of conventional and novel risk factors. Finally, a prospective cohort study in West London found that calcium score was superior to established risk factors in predicting the presence of silent myocardial ischemia on perfusion scans.21 The introduction of coronary CT angiography has changed the field of noninvasive imaging. In addition to existing functional imaging techniques assessing myocardial perfusion and wall motion, CT angiography allows for the detection of both coronary stenoses and calcified and noncalcified plaques. In asymptomatic diabetic individuals, coronary CT angiography revealed a high prevalence of occult CAD (between 64% and 92%) with a high proportion of significant coronary stenosis.22 A German study of 140 asymptomatic diabetic individuals suggests that an atherosclerotic burden score based on the number of diseased coronary segments on coronary CT angiography may significantly improve the risk prediction for CV events over and above conventional risk-factor assessment.23 A study of 313 diabetic patients, mean age 62 years, suggested that the negative predictive value of this imaging modality was excellent, since the mortality was 0% over a mean follow-up of 20 months among those with no evidence of disease.24 The extent of screening for CAD in asymptomatic diabetic patients is a source of controversy. Cardiac testing should be considered in the presence of features of increased CV risk such as peripheral or cerebrovascular disease, renal disease, albuminuria, abnormal resting ECG, diabetic complications, and both traditional and novel CV risk factors.25 In the DIAD (Detection of Ischemia in Asymptomatic Diabetics) study, 1,123 diabetic participants with no symptoms of CAD were randomly assigned to adenosine stress radionuclide myocardial perfusion imaging or no screening in addition to optimal medical treatment.26 At a mean follow-up of 4.8 years, the cumulative cardiac death or MI was 2.9% (0.6% per year), with no difference between the two groups. In the screened group, participants with normal results (N = 409) or small defects (N = 50) had lower event rates than the 33 patients with moderate or large defects on perfusion imaging (0.4% per year vs. 2.4% per year [HR 6.3, P = 0.001]) (Fig. 3-5). Nevertheless, the positive predictive value of having moderate or large defects on perfusion scans was only 12%. The overall rate of coronary revascularization was low in both groups (5.5% in the screened group and 7.8% in the unscreened group). The authors concluded that more aggressive screening for CAD did not improve the outcome of asymptomatic diabetic patients over optimal medical therapy and lifestyle modification. However, because the event rates were lower than estimated, the study was underpowered. In addition, among 33 patients with moderate to large perfusion defects detected by screening, the rate of coronary angiography was only 15%.
TABLE 3-3 Summary of Studies Using Stress Testing in the Diagnosis of Suspected CAD in Diabetic Patients
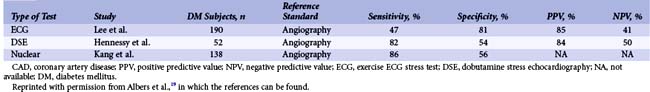
TABLE 3-4 Summary of Studies Using Stress Testing in the Diagnosis of CAD in Asymptomatic Diabetic Patients
 Revascularization in Diabetic Patients with Stable Coronary Disease
Revascularization in Diabetic Patients with Stable Coronary Disease
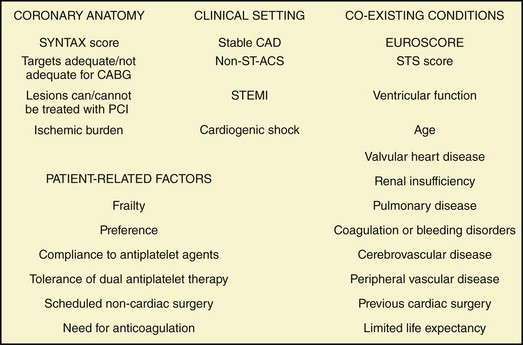
Nearly 1.5 million coronary revascularization procedures, either CABG or PCI, are performed each year in the United States, and approximately one-quarter of them involve diabetic patients.27 Despite improvements in the management of diabetic patients undergoing coronary revascularization—both from a pharmacological and medical device standpoint—diabetes remains an independent predictor of CV events following percutaneous and surgical revascularization. Until recently, comparative data between medical management and revascularization for the diabetic population were sparse. Similarly, little data—mainly derived from subgroup analyses of trials initiated in the late 1980s and early 1990s—were available on the safety and efficacy of PCI versus CABG. However, high-quality comparative data are now available on medical management versus revascularization and on PCI with drug-eluting stents (DESs) versus CABG in diabetic patients. For the choice of revascularization in the individual diabetic patient, several parameters should be taken into account, such as clinical presentation (ACS vs. stable CAD), coronary anatomy, left ventricular function, coexisting conditions, and patient preference (Fig. 3-6).
Percutaneous Coronary Intervention
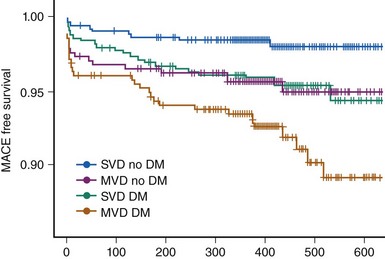
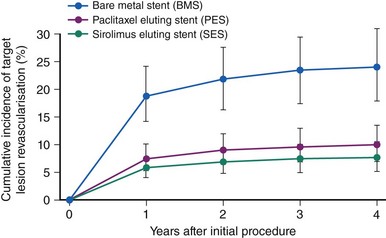
While in-hospital and 30-day outcomes after PCI in diabetic patients have frequently been found to be comparable with those in their nondiabetic counterparts, invariably diabetes remained associated with increased target-vessel revascularization (TVR), major adverse cardiovascular and cerebrovascular events (MACCE), and late mortality. Although stenting has definitely improved the outcomes of diabetic patients undergoing PCI compared with balloon angioplasty, in-stent restenosis remained a challenge for PCI. Accordingly, the outcomes of diabetic patients remained unfavorable in large-scale registries and subgroup analyses of clinical trials compared with those of their nondiabetic counterparts despite a broad use of bare metal stents (BMSs). A metanalysis of six trials with BMSs published up to 2002, including 1,166 diabetic and 5,070 nondiabetic patients, identified diabetes as an independent predictor of restenosis (odds ratio [OR] 1.3), and the restenosis rate in diabetic patients was 37%.28 Similar observations were made in a restenosis trial including a large diabetic population (N = 2,694) undergoing BMS implantation, the PRESTO (Prevention of REStenosis with Tranilast and its Outcomes) study.29 No difference in in-hospital events was observed between diabetic and nondiabetic patients. But after adjusting for baseline characteristics, diabetes was identified as independent predictor of death (relative risk [RR] 1.9) and TVR (RR 1.3) at 9 months. Drug-eluting stents have revolutionized the field of interventional cardiology by dramatically reducing the incidence of restenosis and, as a consequence, of the need for TVR. However, even in the DES era, diabetic patients have worse outcomes compared with those who are nondiabetic. The EVASTENT matched multicenter cohort registry enrolled 1,731 patients undergoing DES implantation (sirolimus-eluting stent, Cypher, Cordis). For each diabetic patient enrolled (stratified as single- or multiple-vessel disease), a nondiabetic patient was subsequently included.30 The median follow-up was 465 days, and 1-year follow-up was available for 98.5% of patients. The worst outcomes were observed among diabetic patients with multivessel disease, while diabetic patients with single-vessel disease had outcomes similar to those of nondiabetic individuals with multivessel disease (Fig. 3-7). Overall, diabetic patients had higher 1-year mortality, stent thrombosis, and TVR rates, and the group at higher risk were diabetic patients treated with insulin. With respect to DES in diabetic patients, an initial subgroup analysis on four DES-versus-BMS trials including 428 patients showed that DES implantation was associated with a statistically significant increase in CV mortality at 4 years (adjusted HR 3.0).31 However, a harmful effect of DESs in diabetic patients could not be reproduced in subsequent studies that were adequately powered. A network metanalysis of 35 randomized trials comparing DES with BMS and including 3,852 diabetic patients showed that DESs, while not affecting overall mortality or MI rates in diabetic patients, were associated with a sizable reduction in target-lesion revascularization (TLR), with a relative RR of 60% to 70% (depending on the type of stent used) and absolute RR of ~16% (Fig. 3-8).32 An analysis of all patients undergoing PCI in nonfederal hospitals in Massachusetts between April 2003 and September 2004 included 5,051 diabetic patients and allowed for a comparison of two propensity-matched diabetic cohorts of 1,476 patients each.33 While the unadjusted cumulative incidence of mortality at 3 years was 14.4% in the DES group and 22.2% in the BMS group (P < 0.001), the corresponding risk-adjusted mortality, MI, and TVR rates at 3 years in propensity-matched cohorts were 17.5% versus 20.7% (P = 0.02), 13.8% versus 16.9% (P = 0.02), and 18.4% versus 23.7% (P < 0.001), respectively. Despite these encouraging results, concerns persist over the risk of DES thrombosis in diabetic patients. Accordingly some trials and registries, albeit not all, have identified diabetes mellitus and particularly insulin-requiring diabetes as an independent predictor of late DES thrombosis. In the previously described EVASTENT matched cohort registry, the 1-year stent thrombosis rate was 3.5% and 1.8% (P < 0.033) in diabetic and nondiabetic patients, respectively.30 Among diabetic individuals, stent thrombosis occurred in 4.3% in the presence of multivessel disease and in 2.3% in single-vessel disease, while in nondiabetic individuals the corresponding rates were 3.0% and 0.8% (P = 0.03). Insulin-requiring diabetes was identified as independent predictor of stent thrombosis (OR 2.9, P = 0.004). Similarly, in a large trial of patients undergoing PCI for ACS, approximately half were treated with DESs and half with BMSs. At a median follow-up of 14 months, the rate of stent thrombosis was 2.8% and 1.4% (HR 2.0; P < 0.0001) respectively in diabetic (N = 3,146) and nondiabetic (N = 10462) individuals.34 In the diabetic subgroup, the rates of stent thrombosis for patients treated with insulin (N = 776) and those on oral hypoglycemic drugs were 3.7% and 2.5%, respectively. Sufficient comparative data among DESs for diabetic patients are available only for the first-generation devices, namely the sirolimus-eluting stent Cypher (Cordis) and the paclitaxel-eluting stent Taxus (Boston Scientific). A metanalysis of five head-to-head studies dedicated to a diabetic patient population (total N = 1,173) demonstrated that the sirolimus-eluting stent was significantly more effective with respect to TLR (5.1% vs. 11.4%; OR 0.41, P < 0.001) and angiographic binary restenosis (5.6% vs. 16.4%; OR 0.30, P < 0.001) compared with the paclitaxel-eluting stent. With respect to cardiac death (2.2% vs. 2.9%), MI (1.5% vs. 2.6%), and stent thrombosis (0.6% vs. 1.2%), no statistically significant differences were identified.