William T. Abraham
Devices for Monitoring and Managing Heart Failure
In the year 2001 a new era of implantable device therapies for the management of heart failure was initiated with U.S. Food and Drug Administration (FDA) approval of the first device for cardiac resynchronization therapy (CRT). Over the subsequent few years, implantable cardioverter-defibrillators (ICDs) and combined CRT-ICD devices were also approved by the FDA for the management of heart failure. ICDs became indicated for the primary prevention of all-cause mortality through a reduction in the incidence of sudden cardiac death (SCD) in patients with heart failure and reduced ejection fractions. Combined CRT-ICD devices were shown to reduce morbidity and mortality in heart failure patients with a reduced ejection fraction (HFrEF) and ventricular dyssynchrony, with a suggestion of additive benefit over a CRT device alone. In acknowledgement of the evidence-based benefits of these devices, the 2005 update of the American College of Cardiology/American Heart Association (ACC/AHA) heart failure guideline strongly supported, with class I indications, the use of ICD and/or CRT devices for the management of eligible heart failure patients1; these indications were updated in 20132 (see Table 26G-1).
In addition to these therapeutic devices, implantable devices that monitor physiologic parameters such as patients’ activity level, heart rate variability (HRV), intrathoracic impedance, and/or hemodynamics have been developed. In some cases these data are already available in currently implantable CRT and ICD devices. The usefulness of such device-based diagnostic or monitoring information is unknown and currently under investigation. This chapter reviews the use of CRT and ICDs for the management of heart failure and discusses the potential usefulness of implantable heart failure monitoring devices. Medical management of heart failure is discussed in Chapters 25 and 27.
Ventricular Dyssynchrony: the Target of Cardiac Resynchronization Therapy
Several conduction abnormalities are commonly seen in association with chronic heart failure. Among these are abnormalities in ventricular conduction, such as bundle branch blocks, that alter the timing and pattern of ventricular contraction such that the already failing heart is placed at further mechanical disadvantage. These ventricular conduction delays produce suboptimal ventricular filling, a reduction in left ventricular contractility, prolonged duration of mitral regurgitation, and paradoxical septal wall motion.3,4 Taken together, these mechanical manifestations of altered ventricular conduction have been termed ventricular dyssynchrony. Ventricular dyssynchrony has been defined by a prolonged QRS duration, generally greater than 120 milliseconds, on the surface electrocardiogram. By this definition, approximately a third of patients with systolic heart failure have ventricular dyssynchrony. In addition to reducing the ability of the failing heart to eject blood, ventricular dyssynchrony has also been associated with increased mortality in heart failure patients.3,4
Ventricular dyssynchrony may now be addressed with pacing therapy through the implantation of pacing leads in both the right and left ventricles. This form of pacing therapy has come to be known as CRT. Favorable single-case experience with CRT in the mid-1990s led to small observational studies evaluating the acute effects of CRT on hemodynamics and other measures of cardiac performance.5 These studies provided additional proof of concept supporting the use of CRT. Several uncontrolled or unblinded studies soon followed to further evaluate the acute and longer-term effects of CRT on clinical status in heart failure patients.5 The results of these trials were equally encouraging, with patients demonstrating consistent, sustained improvement in exercise tolerance, quality of life, and New York Heart Association (NYHA) functional class. Finally, large-scale randomized controlled trials confirmed the beneficial effects of CRT on functional status and outcomes, thereby leading to the initial indications for this therapy. More recent trials have both expanded and also begun to limit the indications for CRT. Ongoing trials are exploring additional potential indications for CRT.
Randomized Controlled Trials of Cardiac Resynchronization Therapy in Patients with New York Heart Association Class III and IV Heart Failure
More than 4000 patients have been evaluated in randomized controlled trials of CRT for NYHA functional class III and IV heart failure. The following randomized controlled trials are considered among the landmark studies of CRT in this patient population: the MUSTIC (Multisite Stimulation in Cardiomyopathy) studies,6,7 the MIRACLE (Multicenter InSync Randomized Clinical Evaluation) trial,8,9 the MIRACLE ICD trial,10 the CONTAK CD trial,11 the CARE-HF (Cardiac Resynchronization in Heart Failure) trial,12,13 and the COMPANION (Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure) trial.14,15 To understand the clinical benefits, risks, and limitations of CRT with or without an ICD, these studies are reviewed.
Multisite Stimulation in Cardiomyopathy Trials
The MUSTIC trials were designed to evaluate the safety and efficacy of CRT in patients with advanced heart failure, ventricular dyssynchrony, and either normal sinus rhythm6 or atrial fibrillation.7 They represent the first randomized single-blind trials of CRT for heart failure. The first study involved 58 randomized patients with NYHA class III heart failure, normal sinus rhythm, and a QRS duration of at least 150 milliseconds. All patients had a CRT device implanted and, after a run-in period, were randomly assigned to either active pacing or no pacing. After 12 weeks, patients crossed over and remained in the alternate study assignment for 12 weeks. The second MUSTIC study included fewer patients (only 37 completers) with atrial fibrillation and a slow ventricular rate (either spontaneously or as a result of radiofrequency ablation). A VVIR biventricular pacemaker and leads for each ventricle were implanted and the same randomization procedure described above was applied; however, biventricular VVIR pacing and single-site right ventricular VVIR pacing (rather than no pacing) were compared in this group of patients with atrial fibrillation.
The primary endpoints of the MUSTIC trials were exercise tolerance as assessed by measurement of peak Vo2 or the 6-minute hall walk test and quality of life as determined by using the Minnesota Living with Heart Failure (MLWHF) questionnaire. Secondary endpoints included rehospitalization and/or modifications in drug therapy for worsening heart failure. Results from the normal sinus rhythm arm of the MUSTIC trials provided strong evidence of benefit. The mean distance walked in 6 minutes was 23% greater with CRT than without CRT (P < 0.001). Significant improvement was also seen in quality of life and NYHA functional class ranking. In addition, fewer hospitalizations occurred during active resynchronization therapy. The atrial fibrillation cohort evaluated in the MUSTIC trials demonstrated similar improvements, although the magnitude of benefit was slightly less.
Multicenter InSync Randomized Clinical Evaluation
MIRACLE was the first prospective, randomized, double-blind, parallel-controlled clinical trial designed to evaluate the benefits of CRT.8,9 Primary endpoints were NYHA class, quality-of-life score (using the MLWHF questionnaire), and 6-minute hall walk distance. Secondary endpoints included assessment of a composite clinical response, cardiopulmonary exercise performance, cardiac structure and function, a variety of measures of worsening heart failure, and combined morbidity and mortality.
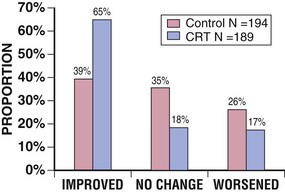
The MIRACLE trial was conducted between 1998 and 2000. It included 453 patients with moderate to severe symptoms of heart failure associated with a left ventricular ejection fraction (LVEF) of 35% or less and a QRS duration of at least 130 milliseconds. Patients were randomly assigned (double-blind) to CRT (n = 228) or to a control group (n = 225) for 6 months while conventional therapy for heart failure was maintained. When compared with the control group, patients randomly assigned to CRT demonstrated a significant improvement in quality-of-life score (−18.0 versus −9.0 points, P = 0.001), 6-minute walk distance (+39 versus +10 meters, P = 0.005), NYHA functional class ranking (−1.0 versus 0.0 class, P < 0.001), treadmill exercise time (+81 versus +19 seconds, P = 0.001), peak Vo2 (+1.1 versus 0.1 mL/kg/min, P < 0.01), and LVEF (+4.6% versus −0.2%, P < 0.001). Patients assigned to CRT demonstrated highly significant improvement in a composite clinical heart failure response endpoint relative to control subjects, thus suggesting an overall improvement in clinical heart failure status (Fig. 26-1). In addition, when compared with the control group, fewer patients in the CRT group required hospitalization (8% versus 15%) or intravenous medications (7% versus 15%) for the treatment of worsening heart failure (both P < 0.05). In the resynchronization group, the 50% reduction in hospitalization was accompanied by a significant reduction in length of stay, which resulted in a 77% decrease in total days hospitalized over a period of 6 months in comparison to the control group. The major limitation of the therapy was unsuccessful implantation of the device in 8% of patients. The results of this trial led to U.S. FDA approval of the InSync system in August 2001, the first approved CRT system in the United States, thus allowing the introduction of CRT into clinical practice.
The MIRACLE trial also provided persuasive evidence supporting the occurrence of reverse left ventricular remodeling with chronic CRT. In the MIRACLE trial, serial Doppler echocardiograms were obtained at baseline and at 3 and 6 months in a subset of 323 patients. CRT for 6 months was associated with reduced end-diastolic and end-systolic volumes (both P < 0.001), reduced left ventricular mass (P < 0.01), increased ejection fraction (P < 0.001), reduced mitral regurgitant blood flow (P < 0.001), and improved myocardial performance index (P < 0.001) in comparison to the control group. These effects are similar to those noted with beta blockade for heart failure but were seen in the MIRACLE trial in patients already receiving beta blocker therapy.
Multicenter InSync Implantable Cardioverter Defibrillator Randomized Clinical Evaluation
The MIRACLE ICD study was designed to be almost identical to the MIRACLE trial. MIRACLE ICD was a prospective, multicenter, randomized, double-blind, parallel-controlled clinical trial intended to assess the safety and efficacy of a combined CRT-ICD system in patients with dilated cardiomyopathy (LVEF ≤35%, left ventricular end-diastolic dimension [LVEDD] ≥55 mm), NYHA class III or IV heart failure, ventricular dyssynchrony (QRS ≥130 milliseconds), and an indication for an ICD.10 Primary and secondary efficacy measures were essentially the same as those evaluated in the MIRACLE trial but also included measures of ICD function.
Of 369 patients receiving devices and randomly assigned, 182 were controls (ICD activated, CRT inactive) and 187 were in the resynchronization group (ICD activated, CRT active). At 6 months, patients assigned to active CRT had a greater improvement in median quality-of-life score (−17.5 versus −11.0, P = 0.02) and functional class (−1 versus 0, P = 0.007) than did controls but were no different from controls in the change in distance walked in 6 minutes (55 versus 53 meters, P = 0.36). Peak oxygen consumption increased by 1.1 mL/kg/min in the resynchronization group versus 0.1 mL/kg/min in controls (P = 0.04), whereas the duration of treadmill exercise increased by 56 seconds in the CRT group and decreased by 11 seconds in controls (P = 0.0006). The magnitude of improvement was comparable to that seen in the MIRACLE trial, thus suggesting that heart failure patients with an indication for an ICD benefit as much from CRT as do patients without an indication for an ICD. The combined CRT-ICD device used in this study was approved by the FDA in June 2002 for use in patients with NYHA class III and IV systolic heart failure, ventricular dyssynchrony, and an indication for an ICD.
CONTAK CD
The CONTAK CD trial enrolled 581 symptomatic heart failure patients with ventricular dyssynchrony and malignant ventricular tachyarrhythmias, all of whom were candidates for an ICD.11 Following unsuccessful implant attempts and withdrawals, 490 patients were available for analysis. The study did not meet its primary endpoint of a reduction in disease progression, as defined by a composite endpoint of hospitalization for heart failure, all-cause mortality, and ventricular arrhythmia requiring defibrillator therapies, although the trends were in a direction favoring improved outcomes with CRT. However, the CONTAK CD trial did demonstrate statistically significant improvements in peak oxygen uptake and quality of life in the resynchronization group in comparison to control subjects, although quality of life was improved only in NYHA class III and IV patients without right bundle branch block. Left ventricular dimensions were also reduced, and LVEFs increased, as seen in other trials of CRT. Importantly, the improvement noted in peak Vo2 with cardiac resynchronization was again comparable to that observed in the MIRACLE trial. Improvements in NYHA functional class were not observed in this study. The CONTAK CD device was approved by the FDA in May 2002 for use in patients with NYHA class III and IV systolic heart failure, ventricular dyssynchrony, and an indication for an ICD.
Cardiac Resynchronization in Heart Failure Trial
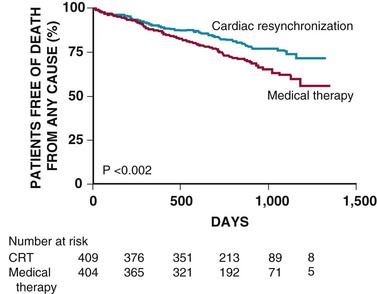
The CARE-HF trial was designed to evaluate the effects of resynchronization therapy without an ICD on morbidity and mortality in patients with NYHA class III or IV heart failure and ventricular dyssynchrony.12,13 In this trial, 819 patients with an LVEF of 35% or less and ventricular dyssynchrony, defined as a QRS duration of 150 milliseconds or greater or a QRS duration between 120 and 150 milliseconds with echocardiographic evidence of dyssynchrony, were enrolled in this randomized, unblinded, controlled trial and monitored for an average of 29.4 months. Of these, 404 patients were randomly assigned to receive optimal medical therapy alone and 409 patients to optimal medical therapy plus resynchronization therapy. The risk for death from any cause or unplanned hospitalization for a major cardiac event, the primary endpoint analyzed as time to the first event, was significantly reduced by 37% in the treatment group versus control subjects (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.51 to 0.77; P < 0.001). In the CRT group, 82 patients (20%) died during follow-up as opposed to 120 patients (30%) in the medical group—a significant 36% reduction in all-cause mortality with resynchronization therapy (HR, 0.64; 95% CI, 0.48 to 0.85; P < 0.002; Fig. 26-2). Resynchronization therapy also significantly reduced the risk for unplanned hospitalization for a major cardiac event by 39%, all-cause mortality plus hospitalization for heart failure by 46%, and hospitalization for heart failure by 52%.
Comparison of Medical Therapy, Pacing, and Defibrillation for Heart Failure
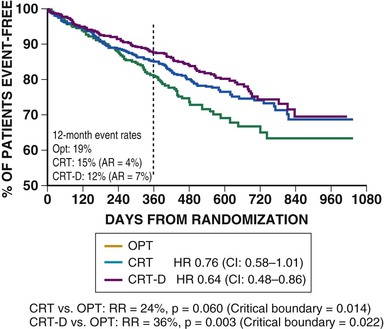
Begun in early 2000, COMPANION was a multicenter, prospective, randomized, controlled clinical trial designed to compare drug therapy alone with drug therapy in combination with cardiac resynchronization in patients with dilated cardiomyopathy, an intraventricular conduction defect, NYHA class III or IV heart failure, and no indication for a device.14,15 The COMPANION trial randomly assigned 1520 patients to one of three treatment groups in a 1 : 2 : 2 allocation—group I (308 patients) received optimal medical care only, group II (617 patients) received optimal medical care and the Guidant CONTAK TR (biventricular pulse generator), and group III (595 patients) received optimal medical care and the CONTAK CD (combined heart failure/bradycardia/tachycardia device). The primary endpoint of the COMPANION trial was a composite of all-cause mortality and all-cause hospitalization, measured as time to the first event beginning from the time of randomization. Secondary endpoints included all-cause mortality and a variety of measures of cardiovascular morbidity. When compared with optimal medical therapy alone, the combined endpoint of mortality or hospitalization for heart failure was reduced by 35% in patients receiving CRT and by 40% in patients receiving CRT-ICD (both P < 0.001). For the mortality endpoint alone, CRT patients had a 24% reduction in risk (P = 0.060) and CRT-ICD patients experienced a 36% reduction in risk (P < 0.003) when compared with optimal medical therapy (Fig. 26-3). The COMPANION trial confirmed the results of earlier resynchronization therapy trials in improving symptoms, exercise tolerance, and quality of life in heart failure patients with ventricular dyssynchrony. In addition, it showed for the first time the impact of CRT-ICD in reducing all-cause mortality and suggested incremental benefit from combined device therapies. These trials in patients with NYHA class III and IV heart failure established the initial 2005 guideline recommendation for CRT: “Patients with LVEF less than or equal to 35%, sinus rhythm, and NYHA functional class III or ambulatory class IV symptoms despite recommended optimal medical therapy and who have cardiac dyssynchrony, which is currently defined as a QRS greater than or equal to 0.120 seconds, should receive CRT, with or without an ICD, unless contraindicated (level of evidence: A).1” These guidelines have recently been updated and are discussed later under the indications for CRT.
More recent clinical trials of CRT have focused on delaying progression of heart failure in asymptomatic or less symptomatic patients. The MIRACLE ICD II trial suggested such a benefit in a small cohort of NYHA class II subjects,16 which led to subsequent large-scale trials in this population.
Randomized Controlled Trials of Cardiac Resynchronization Therapy in Patients with New York Heart Association Class I and II Heart Failure
More than 4500 patients have been evaluated in randomized controlled trials of CRT for those with NYHA functional class I and II heart failure. The following randomized controlled trials are considered to be among the landmark studies of CRT in this patient population: the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial,17,18 MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy),19,20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree