Chagas disease, infection with the parasite Trypanosoma cruzi , has recently been identified as an important emerging parasitic disease in the United States. To describe the cardiac abnormalities in T. cruzi –positive blood donors in southeastern Texas, a pilot study of donors who had screened positive from 2007 to 2012 was performed. This one-time assessment included (1) a questionnaire to evaluate the source of infection, cardiac symptoms, and health co-morbidities; (2) electrocardiography; (3) echocardiography if electrocardiographic findings were abnormal; and (4) measurement of a high-sensitivity troponin T biomarker. Of those with confirmed infection, 41% (7 of 17) had electrocardiographic abnormalities consistent with Chagas cardiomyopathy. In addition, 36% (6 of 17) were suspected to be locally acquired cases. High-sensitivity troponin T serum levels increased with cardiac severity. In conclusion, cardiologists should consider Chagas disease in their differential diagnoses for patients who may have clinically compatible electrocardiographic changes or nonischemic cardiomyopathy, even if the patients have no histories of residing in Chagas-endemic countries.
Highlights
- •
Chagas disease cardiac manifestations were seen in 41% of Texas blood donors during a cross-sectional analysis.
- •
Thirty-six percent of Chagas-positive blood donors were suspected locally acquired cases, putting them at high risk for being mistreated during their follow-up clinical evaluations despite having positive test results from the blood bank.
- •
Blood donors with positive test results for the parasite that causes Chagas disease should be closely monitored for the development of cardiac manifestations.
Chronic Chagas cardiomyopathy is the most common cause of nonischemic cardiomyopathy in Latin America. Up to 30% of patients infected with Trypanosoma cruzi ( T. cruzi ) develop cardiomyopathy characterized by conduction abnormalities, arrhythmias, and left ventricular diastolic and/or systolic dysfunction. Recent rare reports of locally acquired cases and an increase of identified cases due to blood donor screening have highlighted the importance of this disease in the United States. Since blood donor screening began in 2007, approximately 2,000 donors have been identified nationally. However, very little is known about the cardiac outcomes of this presumably healthy blood donor population. The aim of this study was to describe any possible cardiac abnormalities among southeastern Texas blood donors infected with the parasite T. cruzi that causes Chagas disease.
Methods
This study was reviewed and approved by the institutional review boards at Baylor College of Medicine and the Gulf Coast Regional Blood Center. The Gulf Coast Regional Blood Center serves the greater Houston area and >170 hospitals and health care institutions in the 26-county Texas Gulf Coast, Brazos Valley, and East Texas region. Blood donors who tested positive on 2 repeat tests (repeat reactive) on the Ortho T. cruzi enzyme-linked immunosorbent assay system (Ortho-Clinical Diagnostics, Raritan, New Jersey) and radioimmunoprecipitation assay testing (Quest Diagnostics, Madison, New Jersey) were invited to take part in this study through a mailed invitation letter from the blood center. Blood donation testing began at the blood center in 2007. Over the subsequent 5 years, 154 blood donors screened positive for T. cruzi infection. Of these, 30 responded to the invitation letter in the summer of 2013 and choose to take part in this study (20% participation rate). Of those who chose to take part, 17 were confirmed positive by radioimmunoprecipitation assay. The 13 donors who had screened positive but were negative on confirmatory testing served as a control group for the biomarker analysis. Mean time between testing to study assessment was 4 years (range 0.5 to 6).
Research participants took part in a one-time assessment, including (1) a questionnaire to evaluate co-morbidities, travel history, heart-related symptoms, and disease transmission exposures; (2) electrocardiography; (3) echocardiography if electrocardiographic findings were abnormal; and (4) measurement of a high-sensitivity troponin T biomarker. Participants underwent a resting 12-lead electrocardiography performed using a mobile GE Mac 800 machine (GE Healthcare, Waukesha, Wisconsin). Electrocardiograms (ECG) were categorized for Chagas disease severity using the expert panel review criteria derived from the Retrovirus Epidemiology Donor Study–II (REDS-II). Using these criteria, patients were classified into 3 disease categories: (1) major typical ECG abnormalities consistent with Chagas cardiomyopathy, (2) minor ECG abnormalities possibly related to Chagas cardiomyopathy, and (3) indeterminate disease without any related abnormalities. Participants with either major or minor ECG abnormalities consistent with Chagas cardiomyopathy underwent echocardiography performed using a Philips iE33 ultrasound machine (Philips Healthcare, Andover, Massachusetts).
High-sensitivity troponin T (hsTnT) was measured using the Elecys highly-sensitive assay (Roche Diagnostics, Indianapolis, Indiana). HsTnT assays allow the detection of troponin T levels lower than the detection levels of earlier assays. HsTnT is a biomarker of subclinical myocardial injury and has been associated with increased risk for death, cardiovascular mortality, and heart failure in the general population. The limit of blank for the hsTnT assay is 3.0 ng/L, and the limit of detection is 5.0 ng/L. A group of 13 false-positive blood donors served as a control group for this analysis. These 13 donors were confirmed to be negative for T. cruzi infection on 5 additional serologic tests (data not shown).
All statistics were analyzed using Stata version 12 (StataCorp LP, College Station, Texas). Descriptive statistics were performed to evaluate self-reported symptoms of participants by REDS-II Chagas disease severity grouping. Analysis of variance was performed to test for differences in hsTnT biomarker levels between the Chagas disease severity groups and a control group. MapInfo Professional (Pitney Bowes, Stamford, Connecticut) was used to map the location of the participants.
Results
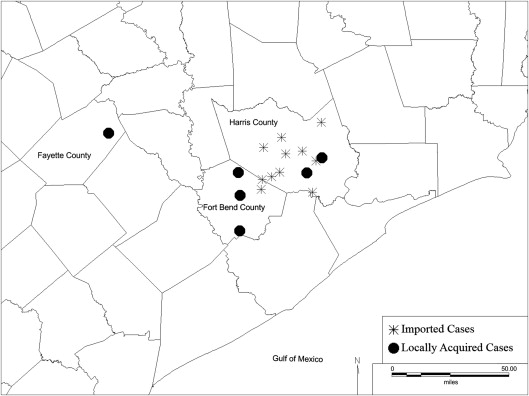
We enrolled 17 blood donors with confirmed T. cruzi infection (Chagas disease) for a one-time assessment of cardiac health. The median age of our study population was 51 years (range 23 to 75), and the population was composed mostly of Hispanic (13 of 17) men (10 of 17). Thirty-six percent (6 of 17) were recognized as potential locally acquired infections. Those with evidence of locally acquired infection were mostly white (4 of 6) and male (4 of 6). One of the 6 participants was classified as a potential locally acquired case because of a report of regularly seeing the insect vector around the participant’s Texas residence and recalling neither having ever been bitten nor seeing the insect vector in the participant’s northern Mexico birth residence. The other 5 locally acquired cases were all in patients born in the United States, without significant histories of travel to endemic countries. Patients with locally acquired cases were more likely to reside in rural counties of southeastern Texas than those whose infections were likely acquired in endemic countries, who tended to reside in urban areas ( Figure 1 ).
T. cruzi –infected donors rarely reported symptoms related to heart disease, which included dyspnea on exertion (2 of 17), heart racing while at rest (3 of 17), and pedal or ankle edema (3 of 17). Diabetes (1 of 17), hypertension (4 of 17), hypercholesterolemia (0 of 17), and history of coronary artery disease (1 of 17) were infrequently reported. In contrast, 41% (7 of 17) had abnormal ECG findings consistent with REDS-II criteria for Chagas cardiac disease ( Table 1 ). Of those with abnormal ECG findings, 72% (5 of 7) had major ECG diagnostic abnormalities consistent with Chagas disease. Additionally, 57% (4 of 7) of those with ECG abnormalities were potentially locally acquired cases. Of those with abnormal ECG results who underwent echocardiography, one individual (14%) had abnormal echocardiographic findings, with an ejection fraction of 40% and global left ventricular dysfunction.
| Age | Race | Gender | Locally Acquired? | Electrocardiogram | Echocardiogram | Relevant Co-Morbidities |
|---|---|---|---|---|---|---|
| 23 | White | Male | Yes | Normal | Not performed | Obesity |
| 33 | Hispanic | Male | No | Normal | Not performed | |
| 38 | Hispanic | Female | No | Normal | Not performed | |
| 40 | Hispanic | Male | No | Normal | Not performed | |
| 45 | Hispanic | Male | No | Possible lateral MI | Normal | |
| 45 | Hispanic | Male | No | Normal | Not performed | |
| 46 | Hispanic | Female | No | Normal | Not performed | |
| 47 | Hispanic | Female | No | Normal | Not performed | |
| 51 | Hispanic | Male | No | Normal | Not performed | |
| 53 | Hispanic | Female | No | SR, RBBB, LAD | Normal | |
| 54 | White | Female | Yes | Normal | Not performed | |
| 58 | Hispanic | Male | No | Non-specific IVCD | Global LV Hypokinesis; EF 40% | |
| 61 | Hispanic | Female | No | Normal | Not performed | |
| 66 | White | Male | Yes | LAFB | Normal | Diabetes; Hypertension |
| 68 | Hispanic | Female | Yes | LVH non-specific T wave abnormality | Normal | Hypertension |
| 75 | White | Male | Yes | SR with 1st degree AV block with inferior-posterior MI | Normal | Hypertension; Coronary Artery Disease |
| 75 | Hispanic | Male | Yes | Atrial paced with RBBB, LAFB, 1st degree AV block | Normal | Hypertension |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


