Atrial fibrillation (AF) is frequently encountered in patients with aortic stenosis (AS) and its incidence also increases with age. In the general population, AF is known to increase cardiovascular risk. We sought to investigate the prognostic importance of AF associated with AS in the context of routine clinical practice. This analysis was based on 809 patients (75 ± 12 years) diagnosed with AS (aortic valve area <2 cm 2 ) and normal (≥50%) ejection fraction (EF). Patients were grouped according to the presence of sinus rhythm (SR) or AF at study enrollment. The AF group comprised 141 patients (17.5%) with AF, whereas 668 patients (82.5%) were in SR at inclusion. Four-year estimates of all-cause mortality with medical and surgical management were 60 ± 5% for the AF group compared with 24 ± 2% for the SR group (p = 0.0001). On multivariate analysis, the risk of all-cause mortality was higher in the AF group than in the SR group (adjusted hazard ratio [HR] 2.47 [1.83 to 3.33], p = 0.0001). AF remained associated with excess mortality risk when the analysis was limited to asymptomatic patients (adjusted HR 2.31 [1.38 to 3.89], p = 0.002) and, respectively, patients with severe AS (adjusted HR 2.22 [1.41 to 3.49], p = 0.001). Among patients managed medically, AF was independently associated with increased risk of death in the overall study population (adjusted HR 2.52 [1.81 to 3.51], p = 0.0001), in asymptomatic AS (adjusted HR 2.12 [1.19 to 3.76], p = 0.01), and in severe AS (adjusted HR 2.23 [1.30 to 3.81], p = 0.004). In conclusion, AF is a major predictor of mortality, in both medically and surgically managed patients with AS, irrespective of the functional status and the severity. AF is, therefore, a strong marker of risk in AS and should be considered for clinical decision making.
Atrial fibrillation (AF) is a common cardiac arrhythmia, occurring especially in aging populations and is associated with increased morbidity and mortality. Valvular heart disease, hypertension, age, male gender, diabetes, heart failure, and coronary artery disease are some of the risk factors that contribute to the onset of AF. The prevalence of AF in patients with AS is high, ranging from 9% in patients with moderate AS to 27% in patients with low-flow, low ejection fraction (EF) AS. Age-related changes in the structure of the heart (left ventricular [LV] hypertrophy, left atrial [LA] dilation, and increased fibrosis) in conjunction with increased chronic afterload induced by the valvular obstacle may explain this over-representation of AF in AS. Indeed, AF might be a marker of chronic pressure and/or volume overload in left-sided valvular disease. Valvular heart diseases are known to be associated with AF, but the prognostic implications of AF in AS remain uncertain. In degenerative mitral regurgitation, the development of AF is associated with a higher rate of adverse cardiac events. In mild-to-moderate AS, the presence of AF is associated with almost threefold increase in the risk of ischemic events. Several studies have suggested that preoperative AF is associated with an adverse prognosis, particularly in low-gradient low-EF AS. We, therefore, investigated the prognostic significance of AF in a large cohort of AS, in the context of routine clinical practice.
Methods
From 2000 to 2012, consecutive patients ≥18 years diagnosed with more than or mild AS (aortic valve calcium with reduction in systolic movements and aortic valve area <2 cm 2 ) and EF ≥50% who were managed medically for at least 3 months after diagnosis were prospectively identified and included in an electronic database. The following patients were excluded: (1) patients with more than mild aortic and/or mitral regurgitation; (2) patients with prosthetic valves, congenital heart disease, supravalvular or subvalvular AS, or dynamic LV outflow tract obstruction; (3) patients with EF <50%; and (4) patients who refused to participate in the study. Eight-hundred ninety-eight patients were enrolled, and 89 patients were subsequently excluded because of missing data (aortic valve area, stoke volume index, or mean Doppler gradient, n = 83) or absence of follow-up (n = 6). Patients were grouped according to the presence of sinus rhythm (SR group) or atrial fibrillation (AF group) on the electrocardiogram at the time of enrollment. A co-morbidity index summating the patient’s individual co-morbidities was calculated. Coronary artery disease was defined as previously described. Institutional review board approval was obtained before conducting the study. The study was conducted in accordance with institutional policies, national legislation, and the revised Declaration of Helsinki. All patients underwent a comprehensive Doppler echocardiographic study, using commercially available ultrasound systems. The echocardiographic and Doppler parameters were measured as previously described. For patients in SR, 3 cardiac cycles were averaged for all measures. For patients in atrial fibrillation, 5 cardiac cycles were averaged. After the initial medical management, treatment was either medical or surgical, as deemed appropriate by the patient’s personal physician. Patients were followed by clinical consultations and echocardiography in the outpatient clinics of the 2 tertiary centers. A few patients were followed in public hospitals or private practices by referring cardiologists working in collaboration with the tertiary centers. Information on follow-up was obtained retrospectively by direct patient interview or by repeated follow-up letters and questionnaires. Median follow-up with medical management was 22.8 months (7 to 53). Median overall follow-up was 39.0 months (11 to 69). The study end point was overall survival after diagnosis starting at the baseline echocardiography and was analyzed with respect to medical management and medical and surgical management. Survival analysis on medical management continued until the last follow-up on medical management (censored at surgery). Survival on medical and surgical management encompassed all medical and surgical management.
Continuous variables are expressed as mean value ± 1 SD, and categorical variables are expressed as frequencies and absolute values. The relation between baseline continuous variables and AF was studied by t tests (for normally distributed variables) or Mann-Whitney U tests (for non-normally distributed variables). Pearson’s chi-square statistic or Fisher’s exact test were used to examine the association between AF and baseline categorical variables. For analysis of outcome on medical treatment, data were censored at the time of cardiac surgery, if performed. The entire follow-up was used to analyze outcome on medical and surgical treatment. Survival rates ± 1 SE of the 2 groups were estimated according to the Kaplan-Meier method and compared by 2-sided log-rank tests. Multivariate analyses of all-cause mortality were performed using Cox proportional hazards models. Model-building techniques were not used, and only those covariates considered to have a potential epidemiologically relevant prognostic impact were entered into the models. These covariates were age, gender, body surface area, co-morbidity index, symptoms at baseline, coronary artery disease, and EF. All p values are the results of 2-tailed tests. Data were analyzed with SPSS 13.0 (SPSS Inc., Chicago, Illinois).
Results
Baseline demographic and clinical characteristics of the 809 patients and the results of the univariate analysis of the comparison between the AF group (141 patients, 17.5%) and the SR group (668 patients, 82.5%) are displayed in Table 1 . On multivariate analysis, older age (odds ratio [OR] 1.07 per year; 95% confidence interval [CI] 1.01 to 1.10; p = 0.02) and greater LA volume (OR = 1.02 per ml; 95% CI 1.004 to 1.0251; p = 0.006) were independently associated with AF.
| Variable | Overall population (n=809) | Sinus rythm ∗ (n=668) | AF ∗ (n=141) | ∗ p value |
|---|---|---|---|---|
| Age (years) | 75 ± 12 | 74 ± 12 | 79 ± 10 | 0.0001 |
| Female gender | 381 (47%) | 302 (45%) | 79 (56%) | 0.02 |
| Body Surface Area (m 2 ) | 1.90 ± 0.23 | 1.90 ± 0.23 | 1.89 ± 0.24 | 0.54 |
| Logistic EuroSCORE (%) | 7.4 ± 7.2 | 6.6 ±6.8 | 11.2 ±8.0 | 0.0001 |
| Coronary artery disease | 265 (33%) | 211 (31%) | 54 (38%) | 0.14 |
| Diabetes mellitus | 243 (30%) | 189 (28%) | 54 (38%) | 0.02 |
| Hypertension | 583 (72%) | 471(70%) | 112 (79%) | 0.04 |
| Systolic blood pressure (mmHg) | 138 ± 20 | 138 ± 20 | 136 ± 21 | 0.19 |
| Diastolic blood pressure (mmHg) | 75±12 | 76±12 | 74±12 | 0.06 |
| New York Heart Association class III-IV | 128 (16%) | 88 (13%) | 40 (28%) | 0.0001 |
| Left ventricular end diastolic diameter (mm) | 49 ± 7 | 49 ± 6 | 50 ± 7 | 0.035 |
| Left ventricular ejection fraction (%) | 64 ± 7 | 64 ± 7 | 63 ± 7 | 0.26 |
| Left ventricular mass index (g/m²) | 118 ± 37 | 117 ± 36 | 125 ± 38 | 0.03 |
| Aortic valve area (cm²) | 1.05 ± 0.37 | 1.05 ± 0.36 | 1.07 ± 0.39 | 0.57 |
| Indexed aortic valve area (cm²/m²) | 0.56 ± 0.20 | 0.55 ± 0.19 | 0.57 ± 0.21 | 0.36 |
| Peak aortic jet velocity (m/s) | 3.6 ± 1.0 | 3.6 ± 0.9 | 3.4 ± 1.0 | 0.05 |
| Valvulo-arterial impedance (mmHg/ml/m²) | 4.3 ± 1.3 | 4.3 ± 1.3 | 4.2 ± 1.3 | 0.49 |
| Mean transvalvular gradient (mmHg) | 33 ± 19 | 34 ± 18 | 31 ± 20 | 0.19 |
| Indexed stroke volume (ml/m²) | 42 ± 11 | 42 ± 10 | 44 ± 11 | 0.42 |
| Left Atrial volume (ml) | 86 ± 32 | 83 ± 30 | 98 ± 36 | 0.002 |
| Systolic pulmonary artery pressure (mmHg) | 33 ± 10 | 33 ± 10 | 37 ± 11 | 0.0001 |
∗ Comparison between sinus rythm group and atrial fibrillation group.
Management was exclusively medical in 588 patients (73%). Aortic valve replacement (n = 219) and transcatheter aortic valve replacement (n = 3) were performed in 221 patients (27%) as decided by the patient’s physician. Aortic bioprostheses were used in 79% of cases (n = 174) and 67 patients underwent at least 1 associated coronary artery by pass graft at the time of surgery. Compared with patients in whom management was solely medical, patients who underwent AVR were younger (70.5 ± 12.6 vs 76.9 ± 11.4 years; p <0.001), more often men (59% vs 51%; p = 0.039), had a lower Charlson index (1.83 ± 1.39 vs 2.44 ± 1.94; p <0.001), smaller AVA (0.87 ± 0.29 vs 1.12 ± 0.37 cm 2 ; p <0.001), higher mean Doppler gradient (44.9 ± 18.3 vs 29.1 ± 16.9 mm Hg; p <0.001), greater indexed LV mass (122.5 ± 38.9 vs 116.9 ± 36.2 g/m²; p = 0.023), and more frequently had coronary artery disease (43% vs 29%; p <0.001).
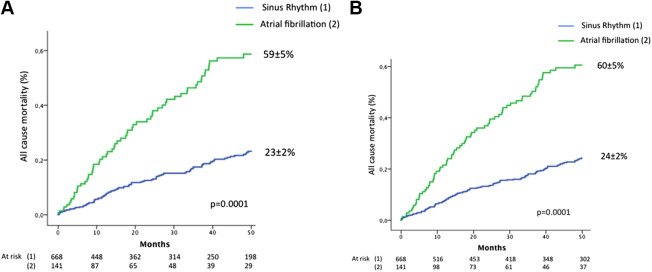
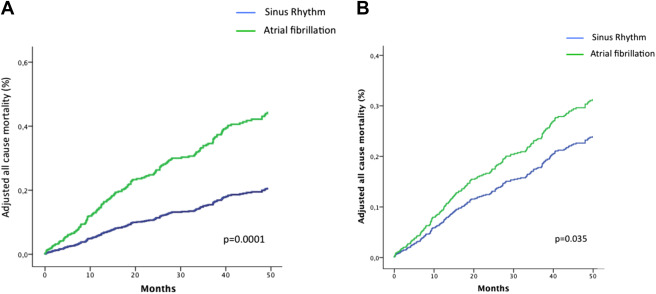
Overall, 4-year all-cause mortality with medical management was higher in the AF group compared with the SR group (59 ± 5% vs 23 ± 2%; log-rank p = 0.0001, Figure 1 ) and with medical and surgical management (60 ± 5% vs 24 ± 2%; p = 0.0001, Figure 1 ). On multivariate analysis ( Figure 2 , Table 2 ), patients with AF exhibited an important excess mortality on medical management and on medical and surgical management.
| Medical management | Medical and surgical management | |
|---|---|---|
| Overall population | Adjusted HR 2.52 [1.81-3.51], p=0.0001 | Adjusted HR 2.47[1.83-3.33], p=0.0001 |
| Severe Aortic stenosis | Adjusted HR 2.23 [1.30-3.81], p=0.004 | Adjusted HR 2.22[1.41-3.49], p=0.001 |
| Asymptomatic patients | Adjusted HR 2.12 [1.19-3.76], p=0.01 | adjusted HR 2.31[1.38-3.89], p=0.002 |
At inclusion, 360 patients (44%) were asymptomatic. The results of the comparison between asymptomatic patients in AF and SR are presented in Table 3 . On multivariate analysis, older age (OR 1.08 per year; 95% CI 1.01 to 1.15; p = 0.017) and greater LA volume (OR 1.02 per ml; 95% CI 1.001 to 1.03; p = 0.05) were independently associated with AF. In this group of asymptomatic patients, overall 4-year mortality with medical management ( Figure 3 ) and with medical and surgical management ( Figure 3 ) remained higher for the AF group. This excess risk associated with AF persisted after adjustment for covariates for medical management and for medical and surgical management ( Table 2 ).
| Variable | Overall population (n=360) | Sinus rythm ∗ (n=276) | AF ∗ (n=84) | ∗ p value |
|---|---|---|---|---|
| Age (years) | 73 ± 13 | 72 ± 14 | 79 ± 9 | 0.0001 |
| Female gender | 145 (40%) | 125 (45%) | 20 (24%) | 0.30 |
| Body Surface Area (m²) | 1.89 ± 0.23 | 1.89 ± 0.23 | 1.89 ± 0.22 | 0.83 |
| Logistic EuroSCORE (%) | 5.4 ± 6.8 | 5.4 ±6.6 | 7.7 ±7.0 | 0.007 |
| Coronary artery disease | 107 (30%) | 90 (33%) | 17 (20%) | 0.10 |
| Diabetes mellitus | 100 (28%) | 86 (31%) | 14 (17%) | 0.39 |
| Hypertension | 251 (70%) | 215 (78%) | 36 (42%) | 0.02 |
| Systolic blood pressure (mmHg) | 138 ± 20 | 138 ± 19 | 138 ± 20 | 0.71 |
| Diastolic blood pressure (mmHg) | 76±13 | 76±12 | 76±13 | 0.87 |
| Left ventricular end diastolic diameter (mm) | 48 ± 7 | 48 ± 6 | 49 ± 7 | 0.19 |
| Left ventricular ejection fraction (%) | 64 ± 7 | 64 ± 7 | 64 ± 8 | 0.47 |
| Left ventricular mass index (g/m²) | 115 ± 38 | 112 ± 36 | 125 ± 41 | 0.008 |
| Aortic valve area (cm²) | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.69 |
| Indexed aortic valve area (cm²/m²) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6± 0.2 | 0.61 |
| Peak aortic jet velocity (m/s) | 3.5 ± 1.0 | 3.6 ± 0.9 | 3.4 ± 1.1 | 0.31 |
| Valvulo-arterial impedance (mmHg/ml/m²) | 4.2 ± 1.3 | 4.2 ± 1.2 | 4.3 ± 1.4 | 0.57 |
| Mean transvalvular gradient (mmHg) | 33 ± 18 | 33 ± 16 | 31 ± 21 | 0.46 |
| Indexed stroke volume (ml/m²) | 42 ± 11 | 42 ± 10 | 41 ± 10 | 0.28 |
| Left atrial volume (ml) | 81 ± 31 | 79 ± 30 | 101 ± 39 | 0.003 |
| Systolic pulmonary artery pressure (mmHg) | 31 ± 8 | 30 ± 8 | 34 ± 9 | 0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


