The present study evaluated progressive cardiac dysfunction using serial circumferential strain (ε cc ) measurements in patients with Duchenne muscular dystrophy (DMD). DMD is characterized by progressive cardiac dysfunction and myocardial fibrosis late in the disease process. We hypothesized that serial ε cc changes could be detected in individual patients with DMD during a time when the left ventricular ejection fraction (EF) changes are insignificant. Cardiac magnetic resonance imaging data from patients with DMD were evaluated. The left ventricular EF was calculated from steady-state free precession cine images and the composite ε cc measurement from tagged cine images. The serial ε cc and EF values for each patient were analyzed using the Wilcoxon sign rank test. Data from 51 patients with DMD (2 studies per patient, mean age at the initial study 11.8 ± 3.5 years, range 7.4 to 25.4) were analyzed, with a mean interval between cardiac magnetic resonance studies of 15.6 ± 6.0 months (range 6.2 to 28.1). In the interval between studies, the ε cc had decreased in all patients with DMD. The average decrease was 1.8 ± 1.3 (p <0.001). However, the EF had decreased in 33 of the 51 patients and had increased in 18 of the 51 patients. On average, the EF decreased by 2.9 ± 8.57% (p = NS). In conclusion, in patients with DMD, ε cc abnormalities indicate progression within a relatively short period when the EF changes were not significant. Serial ε cc measurements might provide reliable monitoring of the progression of DMD-associated cardiac dysfunction before overt heart failure develops, because it is more sensitive than the EF.
At our institution, and others, cardiac magnetic resonance (CMR) imaging with contrast enhancement is routinely used in the surveillance of cardiac global and regional function and for the evaluation of the presence of myocardial fibrosis in patients with Duchenne muscular dystrophy (DMD). We have recently demonstrated that CMR-determined changes in myocardial strain (ε cc ) in a cross-sectional population are a sensitive indicator of abnormality. We also found that these changes become apparent well before the changes detected by traditional functional parameters such as a shortening fraction or ejection fraction (EF). The purpose of the present study was to determine whether myocardial strain values change in a predictable manner during the course of clinically useful periods when tracked in serially. We specifically hypothesized that serial ε cc changes could be detected in individual patients with DMD within a period in which the left ventricular EF changes are not significant.
Methods
All patients with DMD who had undergone serial clinical CMR studies (2 studies per patient) were identified by searching the clinical CMR database at our institution from September 2005 to March 2009. The institutional review board approved the present study. All subjects were ≥5 years of age, eliminating the need for sedation to undergo the CMR study. A diagnosis of DMD was confirmed by skeletal muscle biopsy and/or DNA analysis demonstrating a characteristic dystrophin mutation.
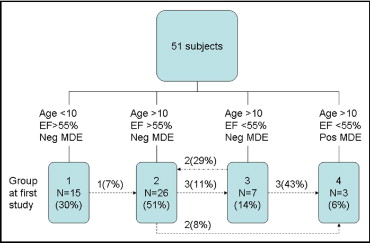
To further determine whether the ε cc changed from the initial strain value, we stratified the subjects into 4 groups according to age, global ventricular function, and the presence of fibrosis, as previously described. Groups 1 through 4 included patients with DMD grouped according to age, EF, and myocardial-delayed enhancement ( Figure 1 ). We have previously shown in a cross-sectional study of normal control subjects that the ε cc is not age dependent; therefore, we did not perform serial CMR studies on the control group. The groups were defined as group 1, patients with DMD ≤10 years old with a normal EF; group 2, patients with DMD >10 years old with a normal EF; group 3, patients with DMD >10 years old with a low EF but no myocardial delayed enhancement; and group 4, patients with DMD >10 years old with a low EF and positive myocardial delayed enhancement) similar to groups B to E in a previous publication.
The CMR studies were conducted using either a Siemens 3 Tesla Trio (Siemens Medical Solutions, Erlangen, Germany) or a GE 1.5 Tesla Signa Excite (General Electric Healthcare, Milwaukee, Wisconsin) system. The type and field strength of the magnetic resonance imaging machine used was based solely on clinical availability, scheduled independent of the patient’s clinical status or previous type or field strength of the first study.
Standard cardiac functional imaging was performed with a retrospectively vectorcardiographic-gated, segmented, steady-state, free precession technique (QMASS MR, Medis Medical Imaging Systems, Leidin, The Netherlands) after localized shimming and/or frequency adjusting. Subjects held their breath as tolerated; for those subjects who could not adequately hold their breath, a free breathing technique with multiple signal averaging was used. Standard functional imaging included a short-axis stack of cine steady-state, free precession images from the cardiac base to the apex. The short axis was prescribed as the perpendicular plane to the left ventricular long axis in the 2- and 4-chamber views, according to previously published protocols. The typical scan parameters were as follows: field of view 32 to 38 cm, slice thickness 5 to 6 mm, gap 1 to 2 mm, number of excitations 1 for breath hold (3 to 4 for free breathing), echo time 1.4 ms and repetition time 2.8 ms for Siemens and echo time 2.0 ms and repetition time 4.0 ms for GE, and in-plane resolution 1.2 to 2.2 mm. A minimum of 12 slices were performed, with 20 phases/slice. The typical temporal resolution of the cine steady-state, free precession images was 30 to 40 ms and was adjusted according to the patient’s heart rate and ability to breath-hold.
Tagged cine magnetic resonance images (HARP, Diagnosoft, Cary, North Carolina) were acquired in the short axis of the mid-ventricle at the level of the papillary muscles based on the 4-chamber view using an electrocardiographic-triggered segmented k-space fast gradient echo sequence with spatial modulation of magnetization in orthogonal planes. The grid tag spacing was 8 mm. The scan parameters used were echo time 3 ms, repetition time 15 ms, field of view (30 to 32) × (25 to 26) cm, slice thickness 6 mm, acquisition matrix 256 × 144, flip 20°, and views per segments 8 to 9. Only quality tagged cine magnetic resonance images were included for analysis (confirmed by 2 independent expert readers, RJF and KNH).
The ventricular volumes, mass, and global function were assessed using standard planimetry techniques and semiautomated computer software (QMASS MR, version 6.1.5, Medis Medical Imaging Systems) by expert readers (KNH, WMG, RF). This assessment was performed using Digital Imaging and Communications in Medicine images from either scanner, independent of the vendor or field strength. The ventricular volumes, mass, and EF were tabulated for each subject and then exported to a spreadsheet file.
As previously described, the tagged images from 2 time points of the mid-ventricle were analyzed using the HARmonic Phase (HARP, Diagnosoft) technique. Only the mid-ventricular slice was analyzed, from our experience, and others’, of the limited reproducibility of the basal and apical slices. Details of the ε cc analysis have been previously described by Hor et al. The ε cc data were exported to a spreadsheet file for analysis. An average of all the regional values per subject was calculated as a composite regional strain value to allow comparison of a single index of the regional strain value. The average ε cc for all subjects was than tabulated for statistical analysis; ε cc is by definition a unitless value defined only as the percentage of change.
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Chicago, Illinois). The data were assessed for normality; because of the failed normality tests, the Wilcoxon sign rank test was performed to assess the serial differences in the means of the ε cc and EF. The mean and SD for the variables of interest were determined for all groups, and p values ≤0.025 by Bonferroni post hoc correction were considered statistically significant.
Results
During the review period, a total of 57 subjects with DMD underwent serial CMR studies with myocardial tagging performed 6 to 28 months apart. Of those, the data from 6 patients was excluded because of inadequate quality (motion artifact) of one of the serial studies. Thus, serial study data from 51 patients were included in the present analysis. Most ambulatory subjects (groups 1 and 2) were treated with systemic steroid therapy. Most nonambulatory subjects (groups 3 and 4) were treated with age-appropriate doses of angiotensin-converting enzyme inhibitors. The retrospective nature of the study precluded controlling for medical therapy.
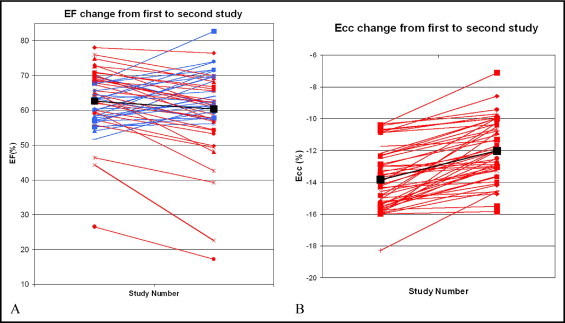
The subject data at the first and second study are listed in Table 1 . The average age of all subjects at the first test was 11.8 ± 3.5 years and at the second test was 13.1 ± 3.5 years (p <0.001). The mean EF for the subjects at the first test was 62.7 ± 8.8% and at the second test was 59.8 ± 11.8% (p = NS). The difference between the mean ε cc at the first test (−13.8 ± 1.9%) differed significantly from that obtained at the second study (−12.0 ± 1.9%; p <0.001).
| Variable | Study Number 1 mean (n = 51) | Study Number 2 mean (n = 51) | p-value |
|---|---|---|---|
| Age (years) | 11.8 ± 3.5 | 13.1 ± 3.6 | <0.001 |
| Ejection fraction | 62.7 ± 8.8% | 59.8 ± 11.8% | NS |
| Mean circumferential strain | −13.8 ± 1.9% | −12.0 ± 1.9% | <0.001 |
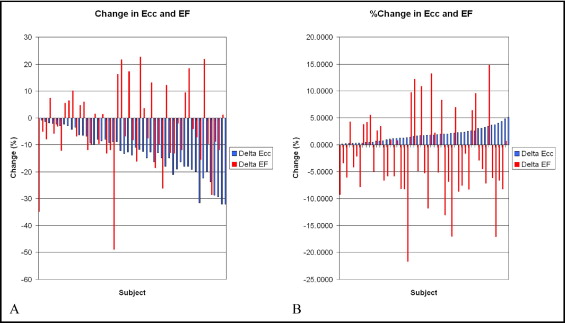
The differences in the serial EF values were variable. For example, 18 (35%) of the 51 patients had a mean increase in the EF of 6.5 ± 4.5% from study 1 to study 2, and 33 had a mean decrease of −8.0 ± 5.3% during the same period. Overall, the mean change in the EF from study 1 to study 2 was −2.9 ± 8.6%, which was not statistically significant (p = NS; Figure 2 ). In contrast, the absolute value of ε cc decreased from the first to the second study in all subjects, regardless of the interval to follow-up. The mean ε cc decreased from study 1 to study 2 by 1.8 ± 1.3%; a relative decrease of 13% compared with the initial study (p <0.001; Figure 2 ).
The variability of the EF values in the face of the consistency of the ε cc values led us to question whether this was partly due to the difference in the scale of the measurements. To examine this, we plotted both the raw changes and the changes as a percentage of the first study value for both EF and ε cc ( Figure 3 ). The ε cc value decreased consistently and the EF demonstrated scattered variation around 0 in both transformations.