A recent histological study of resected scallop-P2 in mitral valve (MV) prolapse, showed that chordae tendinae may be missing or hidden in superimposed fibrous tissue of the leaflets, contributing to their thickening. This may have relevant clinical implication because detailed analysis of MV leaflets has a central role in the evaluation of patients undergoing repair. The aim of this study was to analyze MV leaflets focusing on thickness of prolapsing segments and the presence of chordal rupture (CR). We enrolled 246 patients (age 63 ± 13 years, 72 men) with isolated P2 prolapse and also 50 age-matched patients with normal MV anatomy as control group. Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were retrospectively analyzed to quantify the length and the proximal and distal thickness of both anterior (A2) and posterior (P2) MV scallops. Measurements were performed at end diastole in the standard TTE and TEE views. TTE and TEE measurements were feasible in all cases. Echocardiographically 176 patients had CR (group A), 45 had no rupture (group B), and 25 had an uncertain diagnosis (group C). All pathological groups showed thickening and elongation of involved leaflets versus normal, whereas no differences in leaflets characteristics were found among MV groups. Most patients undergoing MV repair had CR with thickening of the prolapsed segment. These findings are in agreement with recent histological studies showing superimposed fibrous tissue on MV leaflets partially including ruptured chordae. This may also explain that in cases without ruptured chordae, thickness of the leaflets is markedly increased (hidden chordae?). In conclusion, detailed analysis of MV apparatus may further improve knowledge of these patients and may influence surgical timing.
A recent histological study of resected scallop-P2 (middle posterior scallop) in prolapsed mitral valve (MV) showed that chordae tendinae may be missing or hidden in superimposed fibrous tissue of the leaflets, contributing to their thickening. This may explain why the seeing of a chordal rupture (CR) is infrequent but that covered chordae (by fibrous tissue) are nearly universal in patients with MV prolapse (MVP) and mitral regurgitation (MR) severe enough to warrant operative repair strongly suggest that most CRs are clinically silent and occur possibly multiple times through the years. In previous studies the prevalence (by echocardiographic imaging) of CR in patients undergoing MV repair was approximately 55%. Therefore, we may postulate that in several other cases one or more CRs are covered (“hidden”) by the superimposed fibrous tissue on the leaflet’s ventricular surface. From a morphologic and imaging point of view, the prolapsed scallop may appear thickened and the absence of chordae (missing chordae) may be synonymous of earlier CR. Data on MV leaflets thickness in patients with and without CR are limited. The aim of this study in a consecutive series of cases undergoing MV repair was twofold: (1) to analyze MV leaflets focusing on thickness of prolapsing segments and the presence of CR by transthoracic echocardiography (TTE; second harmonic imaging) and transesophageal echocardiography (TEE; natural echo imaging) and (2) to evaluate differences of thickness of the prolapsed scallop versus other scallops in the presence or absence of CR.
Methods
This is a retrospective analysis including 246 consecutive patients with MVP who underwent MV surgery in our hospital from January 2008 to June 2012. All patients have an established diagnosis of severe MR due to degenerative MVP involving P2 (isolated P2 or P2 plus other lesions) evaluated by 2-dimensional (2D) TTE and intraoperative TEE and were suitable for surgical MV repair. In addition, a control group of 50 age-matched patients with normal (NL) MV anatomy undergoing coronary artery bypass was also studied. Exclusion criteria were (1) association of MV stenosis, (2) previous or active endocarditis, (3) aortic valve disease, and (4) myocarditis or pericardial or congenital heart disease. An additional exclusion parameter for the control group was the presence of more than mild MR. Detailed baseline demographic and clinical data were collected. Anatomic features concerning MV were evaluated both using TTE and TEE, in particular length and the proximal and distal thickness of the central scallop of both anterior (A2) and posterior (P2) MV leaflets. Measurements were performed at end diastole in the apical 3-chamber and/or parasternal long-axis views.
The surgical approach for MV repair varied according to MV morphology and to the surgeon’s choice. The procedure was completed with annular ring implantation in all patients. The surgical repair was considered successful in the absence of significant residual MR (more than mild) and/or stenosis (mean diastolic MV gradient >6 mm Hg) and/or systolic anterior motion of the anterior leaflet evaluated by intraoperative TEE. MV replacement was performed only in case of unsuccessful repair.
The local ethics committee approved the study. Informed consent was obtained from all patients.
A complete presurgical TTE was performed in all patients using a Philips iE33 (Philips Medical Systems, Andover, Massachusetts) or a GE Vivid 7 or Vivid 9 (GE Healthcare, Horten, Norway) ultrasound system equipped with S5 or M4S probes, respectively. All images were digitally acquired and stored for offline analysis and included standard 2D, color, pulsed- and continuous-wave Doppler acquisitions. MR was defined as severe when the effective regurgitant orifice area was ≥0.4 cm 2 estimated by proximal isovelocity surface area and/or in the presence of vena contracta width >7 mm or of CR associated with flail leaflets. MVP diagnosis was based on 2D TTE. To assess MV anatomy, we used the Carpentier’s widely recognized nomenclature which divides the posterior leaflet into 3 scallops: lateral (P1), middle (P2), and medial (P3), and the anterior leaflet into 3 segments: lateral (A1), middle (A2), and medial (A3). The anterolateral and posteromedial commissures were also evaluated. All segments were classified as NL, prolapsing (3 mm beyond the annulus plane), or flail. CR was diagnosed as a consistent loss of systolic mitral leaflet coaptation in either the long-axis, apical, 4-chamber, or 2-chamber anterior views, or observation of the flail leaflets in the left atrial cavity. Flail mitral leaflet was defined as the presence of one or both mitral leaflet(s) losing coaptation function and fluttering coarsely in the left atrium during each systole. CR was defined as the presence of the free and highly mobile, linear echoes associated with flail mitral leaflet(s). CR was defined in reference to flail leaflet and scallop(s).
Patients were divided into 3 groups based on the presence of CR (group A), absence of CR (group B), or uncertain CR (group C). The CR was defined uncertain when there was a discordant opinion between 2 observers (see in the following section) and a consensus was not reached. Two-dimensional recordings were made with minimization of gain and optimization of other processing controls to clearly delineate mitral leaflets and chordae without “blooming” of interfaces. Patients were also grouped by the 2 main phenotypes of degenerative MVP, which are Barlow disease (BD) and fibroelastic deficiency (FED). BD is characterized by severe myxomatous degeneration of the leaflets with excess thickened tissue, billowing and/or prolapse of multiple segments of the valve, elongated and thickened chordae, and highly dilated annulus. Conversely, the diagnosis of FED is established in case of NL or even thinner leaflets, no billowing, a single prolapsing segment (usually P2) frequently associated with CR and slight annular dilation.
The length of each leaflet from its hinge point to free edge, but not including the chordae, was traced along the middle of the leaflet on diastolic frames after repeated slow-motion review of the entire cardiac cycle to identify the hinge point and the junction between the leaflet edge and chordae tendineae. Thickness of the leaflets was measured offline with a digital caliper, using ComPACS, version 10.9.1 (Medimatic, Genova, Italy) as the distance from the ventricular to the atrial surface of the leaflet perpendicular to the surface of the leaflet in the apical 3-chamber and/or parasternal long-axis views considering both proximal and distal portion ( Figure 1 ). All images were independently evaluated by 2 experienced cardiologists blinded to clinical data.
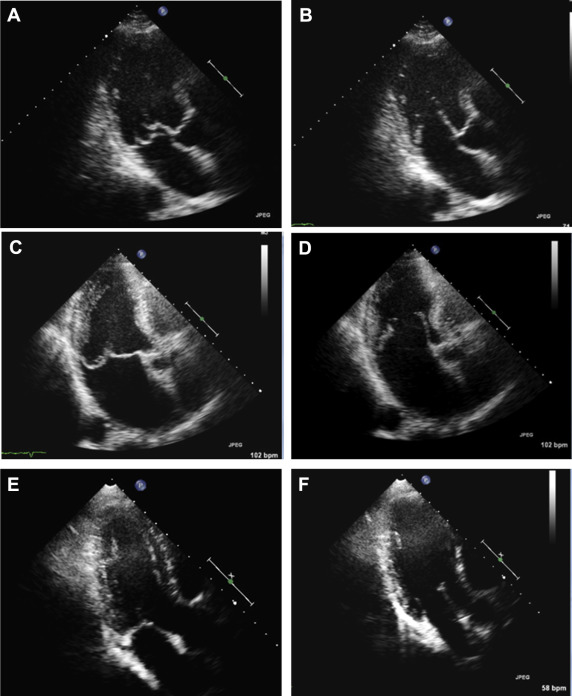
Two-dimensional TEE was performed intraoperatively in all patients after induction of anesthesia. Imaging protocol included complete 2D and/or 3D TEE examinations performed by different experienced operators using an iE33 ultrasound system equipped with X7-2t probe (Philips Medical Systems). Multiplane 2D TEE evaluation included a complete standard protocol for the evaluation of the MV allowing a complete description of morphology of the MV apparatus including CR and evaluation of MR. A systematic evaluation can be obtained using multiple probe locations. Midesophageal planes starting at 0° (fourchamber view), 60° (intercommissural view), and 135° (long-axis equivalent view) should always be obtained since each plane intersects with different components of the valve. Transgastric planes starting with short axis (0° to 20° plane), longitudinal (70° to 80°) showing both papillary muscles and chordal attachments are used with color flow imaging to assess the origins of the regurgitation jets. Three-dimensional TEE was also performed in most cases as a routine practice as previously described to confirm MV morphology and scallops involvement. A complete visualization of the mitral leaflets from their chordal attachments with the papillary muscles to their annular hinge points was obtained in different views. Leaflet length (as from TTE approach) was measured in a diastolic frame in the midesophageal long-axis view. Thickness of MV leaflets was measured in the same view (diastolic frame) in 2 different segments of the leaflet (proximal and distal thickness) ( Figure 2 ).
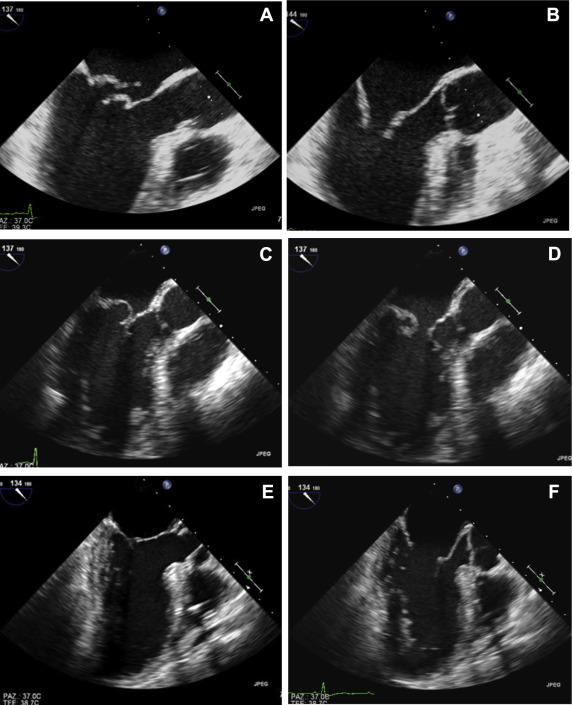
The surgeon described the anatomy of the valve with the same Carpentier classification. CR was annotated.
Continuous variables are presented as mean ± SD and categorical variables as frequencies and relevant percentages. The data distribution normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Continuous variables were compared using the unpaired Student t test or Mann–Whitney U test, and for categorical variables the chi-square test or Fisher’s exact test, as appropriate, for comparison between patients with MVP and patients with NL MV anatomy. To test for differences among group A, group B, group C, and patients with NL MV anatomy, one-way analysis of variance or Friedman test was applied to normally or nonnormally distributed data, respectively. Post hoc analysis for significant results was performed using the Bonferroni correction. The sensitivity, specificity, and accuracy of echocardiographic evaluation of the involved scallops (or CR) were calculated with surgical findings as reference. To assess the reproducibility of the TEE and TTE measurements of leaflet thickness and length, intraobserver and interobserver variability were evaluated in a subset of 30 randomly selected patients with MVP. Each parameter was reevaluated ≥2 weeks later on the same data set by the main investigator and by a second investigator who was blinded to the results obtained by the main investigator. Both intraobserver and interobserver variability are reported in terms of intraclass correlation coefficients (ICCs) and coefficients of variation (CV, percentages). Statistical analyses were conducted using SPSS version 20.0 (SPSS Inc., Chicago, Illinois). Differences were considered statistically significant at p <0.05.
Results
Baseline clinical and echocardiographic data of patients affected by MVP and controls are reported in Table 1 . The mean age of this cohort of 246 patients with severe MR due to MVP involving P2 was 63 ± 13 years and 174 patients (71%) were men. CR was diagnosed by echocardiography in 176 patients (group A), 45 has no CR (group B), and 25 has an uncertain echocardiographic diagnosis (group C). Concerning main echocardiographic data, no differences were found among the 3 pathological groups. Patients with MVP showed increased left ventricular volumes as well as left atrium enlargement and high systolic pulmonary pressure according to the severity of the regurgitation itself.
| All (n = 246) | Group A (n = 176) | Group B (n = 45) | Group C (n = 25) | NL (n = 50) | p 1 | P 2 | |
|---|---|---|---|---|---|---|---|
| Age (Years) | 63±13 | 63±12 | 63±15 | 63±16 | 64±9 | 0.438 | 0.895 |
| Male | 174(71%) | 125(71%) | 29 (64%) | 20(80%) | 42 (84%) | 0.054 | 0.127 |
| BSA (m 2 ) | 1.8±0.2 | 1.8±0.2 ∗ | 1.8±0.2 ∗ | 1.8±0.2 | 1.9±0.2 | 0.001 | 0.003 |
| EDV index (mL/m 2 ) | 76±18 | 77±18 ∗ | 76±17 ∗ | 70±16 ∗ | 52±16 | <0.001 | <0.001 |
| ESV index (mL/m 2 ) | 27±9 | 27±9 | 28±8 | 25±9 | 25±15 | 0.370 | 0.418 |
| EF (%) | 65±7 | 65±7 ∗ | 64±7 ∗ | 65±8 ∗ | 57±10 | <0.001 | <0.001 |
| Atrial Area (cm 2 ) | 30±8 | 30±9 ∗ | 30±8 ∗ | 27±6 ∗ | 20±5 | <0.001 | <0.001 |
| PAPS (mmHg) | 38±11 | 39±12 ∗ | 39±12 ∗ | 33±9 | 28±6 | <0.001 | <0.001 |
Measurements of the leaflets obtained from 2D TTE and TEE in patients with MVP and controls are presented in Table 2 . All pathological groups showed significant thickening and elongation of involved leaflets versus the control group, whereas no differences were found in leaflets characteristics among MV groups. Thickness of both anterior and posterior leaflets in all patients with MVP was significantly greater in comparison to controls. Posterior leaflet was thicker than the anterior one in the 3 MVP groups, whereas no difference was found between thickness of anterior and posterior leaflet in control group. Thickness was found homogeneously higher in the proximal and distal segments of both anterior and posterior leaflets in pathological groups. In controls, the anterior and posterior leaflet thickness was similar. As concerns comparisons between the TTE and TEE measurements, leaflet thickness (proximal and distal segments) resulted higher with the TTE approach in all groups. No significant difference in thickness of P2 was observed in group A or group B as well as in group C. No difference was found among the 3 groups concerning length of the leaflets, whereas in all patients with MVP, leaflets were significantly longer. As concerns BD and FED, both groups had thickening of the leaflets and in BD P2 and A2 leaflet thickness was mildly higher in comparison to FED ( Table 2 ).
| Group A (n = 176) | Group B (n = 45) | Group C (n = 25) | NL (n = 50) | BD (n= 187) | FED (n= 59) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A2 | P2 | A2 | P2 | A2 | P2 | A2 | P2 | A2 | P2 | A2 | P2 | |
| TTE | ||||||||||||
| Proximal thickness [mm] | 3.2±0.7 † | 3.7±0.8 ∗ † | 3.1±0.6 † | 3.7±0.9 ∗ † | 3.0±0.6 † | 4.0±0.8 ∗ † | 1.8±0.5 | 1.8±0.5 | 3.2±0.7 | 3.8±0.9 | 3.1±0.7 | 3.5±0.7 ‡ |
| Distal thickness [mm] | 3.4±1.1 † | 3.9±1.0 ∗ † | 3.3±1.3 † | 3.9±1.0 ∗ † | 3.4±1.1 † | 4.0±1.1 ∗ † | 1.8±0.4 | 1.7±0.5 | 3.5±1.1 | 4.0±1.1 | 3.1±1.0 ‡ | 3.6±0.8 ‡ |
| Length [mm] | 27.4±3.4 † | 22.7±3.5 † | 26.9±3.4 † | 23.1±3.8 † | 26.0±3.0 | 21.3±3.6 | 25.4±3.5 | 20.6±3.2 | 27.6±3.4 | 22.9±3.6 | 25.9±2.9 ‡ | 21.6±3.4 ‡ |
| TEE | ||||||||||||
| Proximal thickness [mm] | 2.6±0.7 † | 3.2±0.8 ∗ † | 2.5±0.6 † | 3.2±0.8 ∗ † | 2.3±0.6 † | 3.1±0.7 ∗ † | 1.7±0.5 | 1.7±0.5 | 2.6±0.7 | 3.2±0.8 | 2.5±0.6 | 3.0±0.7 |
| Distal thickness [mm] | 3.0±0.9 † | 3.3±0.9 ∗ † | 3.0±0.9 † | 3.5±0.7 ∗ † | 3.1±0.9 † | 3.7±0.8 ∗ † | 1.7±0.5 | 1.6±0.5 | 3.1±0.9 | 3.5±0.9 | 2.7±0.7 ‡ | 3.1±0.7 ‡ |
| Length [mm] | 27.1±3.4 † | 21.7±3.5 † | 27.1±3.9 † | 22.1±3.5 † | 26.0±3.5 | 20.3±2.9 | 24.7±2.7 | 20.1±2.9 | 27.3±3.5 | 21.9±3.5 | 25.9±3.4 ‡ | 20.6±3.1 ‡ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


