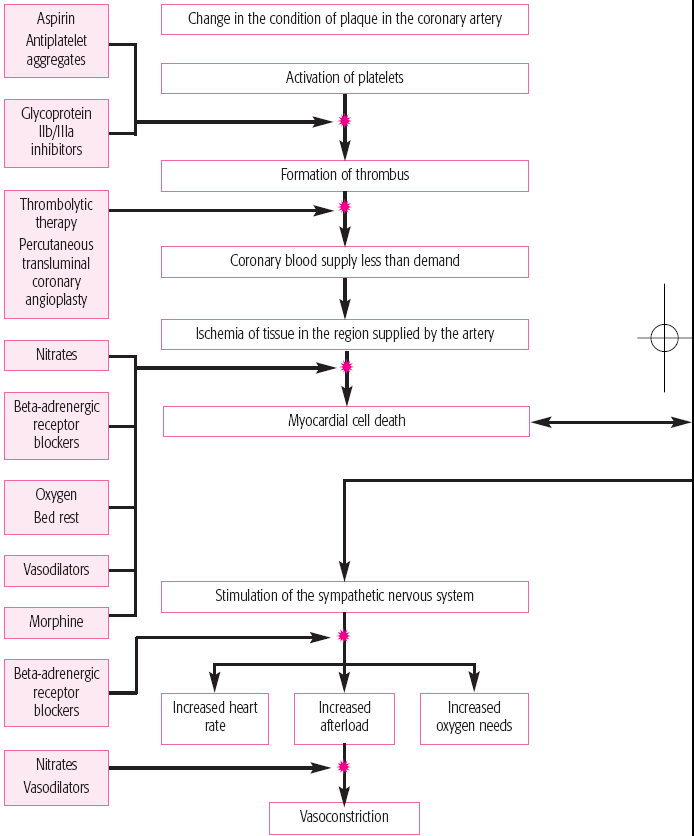
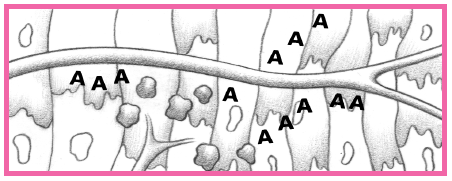
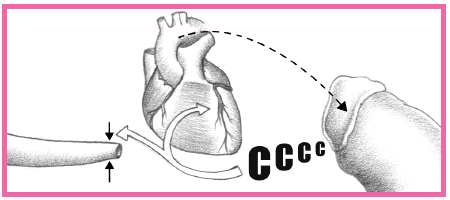
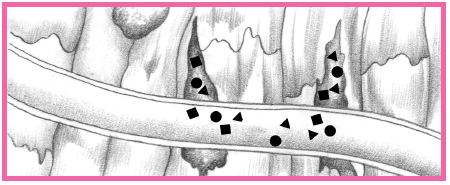
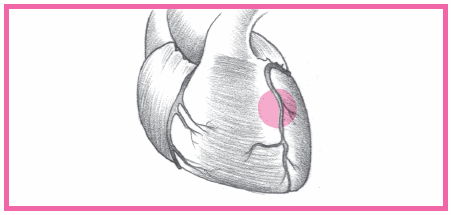
have distal microthrombi that cause necrosis in some myocytes. The smaller vessels infarct, thus placing the patient at higher risk for NSTEMI. If a thrombus fully occludes the vessel for a prolonged time, a STEMI usually develops. (See What happens during an MI, pages 384 and 385.) This type of MI involves a greater concentration of thrombin and fibrin. (See How ACS affects the body, pages 386 and 387.)
 |
Anterior-wall MI occurs when the left anterior descending artery becomes occluded.
Septal-wall MI typically accompanies an anterior wall MI because the ventricular septum is supplied by the left anterior descending artery as well.
Lateral-wall MI is caused by a blockage in the left circumflex artery, and usually accompanies an anterior- or inferior-wall MI.
Inferior-wall MI is caused by occlusion of the right coronary artery; it usually occurs alone or with a lateral-wall or right-ventricular MI.
Posterior-wall MI is caused by occlusion of the right coronary artery or the left circumflex arteries.
Right-ventricular MI follows occlusion of the right coronary artery; this type of an MI rarely occurs alone. (In 40% of patients, a right-ventricular MI accompanies an inferior-wall MI.)
aging
diabetes mellitus
elevated triglyceride, low-density lipoprotein, and cholesterol levels, and decreased high-density lipoprotein levels
excessive intake of saturated fats, carbohydrates, or salt
hypertension
obesity
positive family history of coronary artery disease (CAD)
 |
 |
 |
 |
 |
 |
 |
 |
 |
sedentary lifestyle
smoking
stress or a type A personality (aggressive, competitive attitude, addiction to work, chronic impatience).
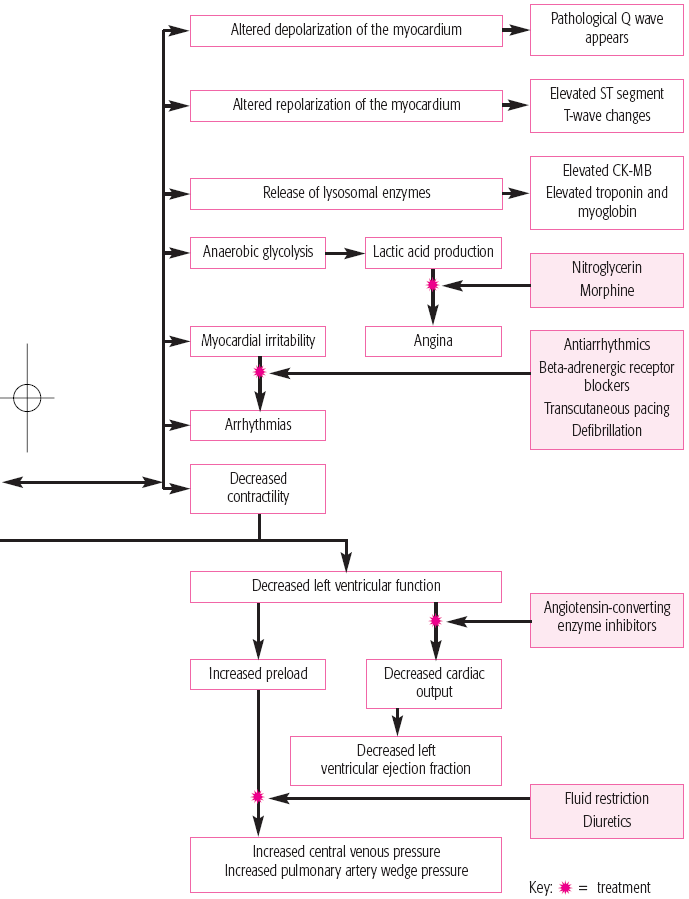
Arrhythmias
Heart failure causing pulmonary edema
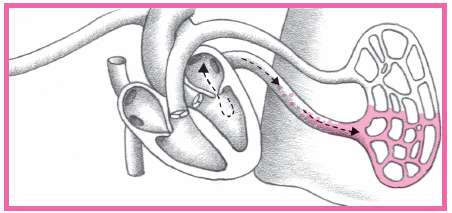
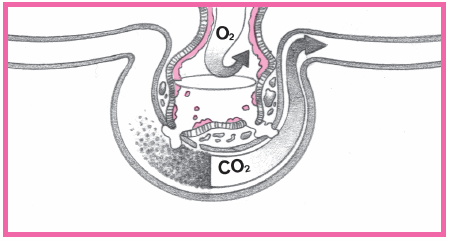
An area of viable ischemic tissue surrounds the zone of injury.
When the heart muscle is damaged, the integrity of the cell membrane is impaired.
Intracellular contents, including cardiac enzymes (such as creatine kinase and aspartate aminotransferase) and proteins (such as troponin T, troponin I, and myoglobin) are released.
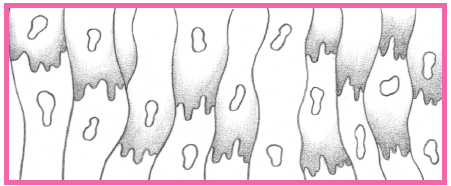
Within 24 hours, the infarcted area becomes edematous and cyanotic.
During the next several days, leukocytes infiltrate the necrotic area and begin to remove necrotic cells, thinning the ventricular wall.
Scar formation begins by the 3rd week after a myocardial infarction (MI); by the 6th week, scar tissue is well established.
The scar tissue that forms on the necrotic area inhibits contractility.
Compensatory mechanisms (vascular constriction, increased heart rate, and renal retention of sodium and water) try to maintain cardiac output.
Ventricular dilation may also occur in a process called remodeling.
Functionally, an MI may cause reduced contractility with abnormal wall motion, altered left ventricular compliance, reduced stroke volume, reduced ejection fraction, and elevated left ventricular end-diastolic pressure.
Cardiogenic shock is caused by failure of the heart to perform as an effective pump and can result in low cardiac output, diminished peripheral perfusion, pulmonary congestion, and elevated systemic vascular resistance and pulmonary vascular pressures.
Ineffective contractility of the heart leads to accumulation of blood in the venous circulation distal to the failing ventricle.
Arrhythmias can occur in the patient with an acute MI as a result of autonomic nervous system imbalance, electrolyte disturbances, ischemia, and slowed conduction in zones of ischemic myocardium.
Hypoperfusion of the brain results in altered mental status, involving changes in the level of consciousness, restlessness, irritability, confusion, or disorientation.
Stupor or coma may result if the decrease in cerebral perfusion continues.
Shock and hypoperfusion from an MI cause the kidney to respond by conserving salt and water.
Poor perfusion results in diminished renal blood flow, and increased afferent arteriolar resistance occurs, causing a decreased glomerular filtration rate.
Increased amounts of antidiuretic hormone and aldosterone are released to help maintain perfusion. Urine formation, however, is reduced.
Depletion of renal adenosine triphosphate stores results from prolonged renal hypoperfusion, causing impaired renal function.
Cardiogenic shock with left-sided heart failure results in increased fluid in the lungs. This process can overwhelm the capacity of the pulmonary lymphatics, resulting in interstitial and alveolar edema.
Lung edema occurs when pulmonary capillary pressure exceeds 18 mm Hg.
Pulmonary alveolar edema develops when pressures exceed 24 mm Hg, impairing oxygen diffusion.
Increased interstitial and intraalveolar fluid causes progressive reduction in lung compliance, increasing the work of ventilation and increasing perfusion of poorly ventilated alveoli.
Cardiogenic shock
Rupture of the atrial or ventricular septum, ventricular wall, or valves
Pericarditis
Ventricular aneurysms
Mural thrombi causing cerebral or pulmonary emboli
Dressler’s syndrome (post-MI pericarditis) occurring days to weeks after an MI and causing residual pain, malaise, and fever (see Complications of an MI, pages 388 to 390)
 AGE AWARE
AGE AWARE
Typically, the patient reports the cardinal symptom of an MI: persistent, crushing substernal pain that may radiate to the left arm, jaw, neck, and shoulder blades. He commonly describes the pain as heavy, squeezing, or crushing, and it may persist for 12 or more hours.
COMPLICATION | ASSESSMENT | TREATMENT |
|---|---|---|
Arrhythmias | • Electrocardiogram (ECG) shows premature ventricular contractions, ventricular tachycardia, or ventricular fibrillation; in an inferior myocardial infarction (MI), bradycardia and junctional rhythms or atrioventricular (AV) block; in an anterior MI, tachycardia or heart block. | • Antiarrhythmic, atropine, cardioversion, defibrillation, and pacemaker |
Heart failure | • In left-sided heart failure, chest X-rays show venous congestion and cardiomegaly. • Catheterization shows increases in pulmonary artery systolic and diastolic pressures, pulmonary artery wedge pressure (PAWP), central venous pressure, and systemic vascular resistance (SVR). | • Diuretic, angiotensin-converting enzyme inhibitor, beta-adrenergic receptor blocker, vasodilator, inotropic, and cardiac glycoside |
Cardiogenic shock | • Catheterization shows decreased cardiac output, increased pulmonary artery systolic and diastolic pressures, decreased cardiac index, increased SVR, and increased PAWP. • Signs are hypotension, tachycardia, decreased level of consciousness, decreased urine output, jugular vein distention, S3 and S4, and cool, pale skin. | • I.V. fluids, vasodilator, diuretic, cardiac glycoside, intra-aortic balloon pump (IABP), vasopressor, and beta-adrenergic receptor stimulant |
Mitral insufficiency | • Auscultation reveals apical holosystolic murmur. • Dyspnea is prominent. • Catheterization shows increased pulmonary artery pressure (PAP) and PAWP. | • Nitroglycerin (Nitrostat), nitroprusside (Nitropress), IABP, and surgical replacement of the mitral valve; possible myocardial revascularization with significant coronary artery disease |
Ventricular septal rupture | • Echocardiogram shows valve dysfunction. • In left-to-right shunt, auscultation reveals a harsh holosystolic murmur and thrill. • Catheterization shows increased PAP and PAWP. • Increased oxygen saturation of right ventricle and pulmonary artery confirms the diagnosis. • Color-flow and Doppler echocardiography demonstrate left-to-right blood flow across the septum. | • Surgical correction (may be postponed, but more patients have surgery immediately or up to 7 days after septal rupture), IABP, nitroglycerin, nitroprusside, low-dose inotropic (dopamine [Inocor]), and cardiac pacing when high-grade AV blocks occur |
Pericarditis or Dressler’s syndrome | • Auscultation reveals a pericardial friction rub. • Chest pain is relieved in sitting position. • Sharp pain is unlike previously experienced angina. | • Anti-inflammatory agent, such as aspirin (Ecotrin) or other nonsteroidal anti-inflammatory drug or corticosteroid |
Ventricular aneurysm | • Chest X-rays may show cardiomegaly. • ECG may show arrhythmias and persistent ST-segment elevation. • Left ventriculography shows altered or paradoxical left ventricular motion. | • Cardiopulmonary resuscitation (CPR), cardioversion, defibrillation (if ventricular tachycardia or fibrillation occurs), antiarrhythmic, vasodilator, anticoagulant, cardiac glycoside, diuretic and, possibly, surgery |
Cerebral or pulmonary embolism | • Dyspnea and chest pain or neurologic changes occur. • Nuclear scan shows ventilation-perfusion mismatch in pulmonary embolism. • Angiography shows arterial blockage. | • Oxygen and heparin • CPR, epinephrine, or cardiac pacing |
Ventricular rupture | • Cardiac tamponade occurs. • Arrhythmias, such as ventricular tachycardia and ventricular fibrillation, occur or sudden death results. | • Resuscitation as per Advanced Cardiac Life Support protocol • Possible emergency surgical repair if CPR successful |
In some patients—particularly elderly patients or those with diabetes—pain may not occur; in others, it may be mild and confused with indigestion.
Patients with CAD may report increasing anginal frequency, severity, or duration (especially when not precipitated by exertion, a heavy meal, or cold and wind).
The patient may also report a feeling of impending doom, fatigue, nausea, vomiting, and shortness of breath. Sudden death, however, may be the first and only indication of an MI.
Women may experience atypical signs and symptoms of an MI, which may go unnoticed. These include:
– burning sensation or discomfort in the upper abdomen
– difficulty breathing
– nausea and vomiting
– weakness or fatigue
– profuse sweating
– light-headedness and fainting.
Inspection may reveal an extremely anxious and restless patient with dyspnea and diaphoresis.
If right-sided heart failure is present, you may note jugular vein distention.
Within the first hour after an anterior MI, about 25% of patients exhibit sympathetic nervous system hyperactivity, such as tachycardia and hypertension.
Up to 50% of patients with an inferior MI exhibit parasympathetic nervous system hyperactivity, such as bradycardia and hypotension.
In patients who develop ventricular dysfunction, auscultation may disclose an S4, an S3, paradoxical splitting of S2, and decreased heart sounds. A systolic murmur of mitral insufficiency may be heard with papillary muscle dysfunction secondary to infarction. A pericardial friction rub may also be heard, especially in patients who have a transmural MI or have developed pericarditis.
Fever is unusual at the onset of an MI, but a low-grade fever may develop during the next few days.
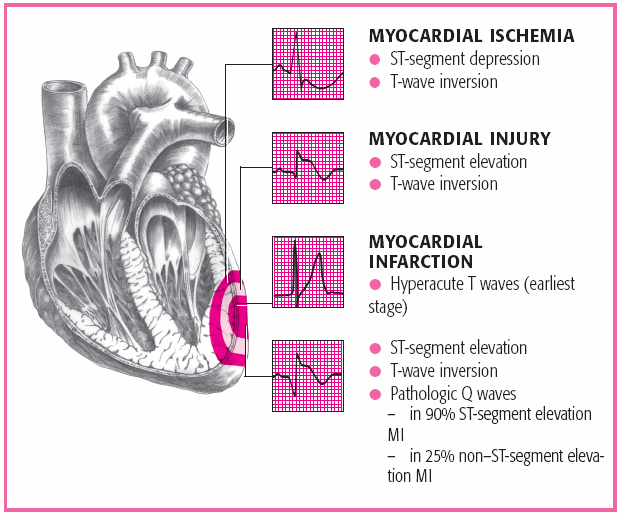
Serial 12-lead electrocardiogram (ECG) readings may be normal or inconclusive during the first few hours after an MI. Characteristic abnormalities include serial ST-segment depression in patients with a subendocardial MI, and ST-segment elevation and Q waves, representing scarring and necrosis, in those with a transmural MI. (See ECG characteristics in ACS, page 392.)
The creatine kinase (CK) level is elevated, especially the CK-MB isoenzyme, the cardiac muscle fraction of CK.
White blood cell count usually appears elevated on the 2nd day and lasts 1 week.
Myoglobin (the hemoprotein found in cardiac and skeletal muscle) is released with muscle damage and may be detected as soon as 2 hours after an MI.
Troponin I, a structural protein found in cardiac muscle, is elevated only in patients with cardiac muscle damage. It’s more specific than the CK-MB level. Troponin levels increase within 4 to 6 hours of myocardial injury and may remain elevated for 5 to 11 days.
Echocardiography shows ventricular-wall dyskinesia with a transmural MI and helps evaluate the ejection fraction.
Nuclear ventriculography (multiple gated acquisition scanning or radionuclide ventriculography) can identify acutely damaged muscle by picking up accumulations of radioactive nucleotide, which appears as a hot spot on the film. Myocardial perfusion imaging with thallium-201 or Cardiolite reveals a “cold spot” in most patients during the first few hours after a transmural MI.
Elevated homocysteine and C-reactive protein levels have been found incidentally in patients with an MI and may indicate a newer risk factor. Folic acid supplementation is used to treat elevated homocysteine levels.
cardiac workload and increasing oxygen supply to the myocardium, preventing major adverse cardiac events, and providing for cardiopulmonary resuscitation and defibrillation when ventricular fibrillation or pulseless ventricular tachycardia (VT) is present. (See Treating an MI, pages 394 and 395.)
Patients with an ST-segment elevation greater than 1 mm in two or more contiguous leads or with new LBBB need to be treated for an acute MI.
More than 90% of patients with this presentation will develop new Q waves and have positive serum cardiac markers.
Repeating the ECG may be helpful for patients who present with hyperacute T waves.
Patients with ST-segment depression indicating a posterior MI benefit most when an acute MI is diagnosed.
Ischemia should be suspected with findings of ST-segment depression greater than or equal to 0.5 mm, marked symmetrical T-wave inversion in multiple precordial leads, and dynamic ST-T changes with pain.
Patients who display persistent symptoms and recurrent ischemia, diffuse or widespread ECG abnormalities, heart failure, and positive serum markers are considered high risk.
A normal ECG won’t show ST-segment changes or arrhythmias.
If the ECG is nondiagnostic, it may show an ST-segment depression of less than 0.5 mm or a T-wave inversion or flattening in leads with dominant R waves.
Continue assessment of myocardial changes through use of serial ECGs, ST-segment monitoring, and serum cardiac markers.
If further assessment is warranted, perform perfusion radionuclide imaging and stress echocardiography.
Obtain a 12-lead ECG and cardiac markers to help confirm the diagnosis of an acute MI. Cardiac markers (especially troponin I and CK-MB) are used to distinguish unstable angina and NSTEMI.
Use the memory aid “MONA,” which stands for morphine, oxygen, nitroglycerin, and aspirin, to treat any patient experiencing ischemic chest pain or suspected ACS. Administer:
– morphine to relieve pain
– oxygen to increase oxygenation of the blood
– nitroglycerin sublingually to relieve chest pain (unless systolic blood pressure is less than 90 mm Hg or heart rate is less than 50 beats/minute or greater than 100 beats/minute)
– aspirin to inhibit platelet aggregation.
a beta-adrenergic receptor blocker to reduce the heart’s workload and oxygen demands
heparin and a glycoprotein IIb/IIIa inhibitor to minimize platelet aggregation and the danger of coronary occlusion with high-risk patients (patients with planned cardiac catheterization and positive troponin)
nitroglycerin I.V. to dilate coronary arteries and relieve chest pain (unless systolic blood pressure is less than 90 mm Hg or heart rate is less than 50 beats/minute or greater than 100 beats/minute)
an antiarrhythmic, transcutaneous pacing (or transvenous pacemaker), or defibrillation if the patient has ventricular fibrillation or pulseless VT
percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG) surgery for obstructive lesions
an antilipemic to reduce elevated cholesterol or triglyceride levels.
thrombolytic therapy (unless contraindicated) within 12 hours of onset of symptoms to restore vessel patency and minimize necrosis in STEMI
I.V. heparin to promote patency in the affected coronary artery
a beta-adrenergic receptor blocker to reduce myocardial workload
an antiarrhythmic, transcutaneous pacing (or transvenous pacemaker), or defibrillation if the patient has ventricular fibrillation or pulseless VT
 |
an angiotensin-converting enzyme inhibitor to reduce afterload and preload and prevent remodeling (begin in STEMI 6 hours after admission or when the patient’s condition is stable)
interventional procedures (such as PTCA, stent placement, or surgical procedures such as CABG) may open blocked or narrowed arteries.
Care for patients who have suffered an MI is directed toward detecting complications, preventing further myocardial damage, and promoting comfort, rest, and emotional well-being. Most patients receive treatment in the coronary care unit (CCU), where they’re under constant observation for complications.
On admission to the CCU, monitor and record the patient’s ECG readings, blood pressure, temperature, and heart and breath sounds.
Assess pain and give an analgesic. Record the severity, location, type, and duration of pain. Don’t give I.M. injections because absorption from the muscle is unpredictable, the CK level may be falsely elevated, and I.V. administration gives more rapid relief of signs and symptoms.
Check the patient’s blood pressure after giving nitroglycerin, especially the first dose.
Frequently monitor the ECG to detect rate changes and arrhythmias. Analyze rhythm strips. Place a representative strip in the patient’s chart if any new arrhythmias are documented, if chest pain occurs, at every shift change, or according to facility protocol.
During episodes of chest pain, obtain a 12-lead ECG (before and after nitroglycerin therapy as well). Also obtain blood pressure and pulmonary artery catheter measurements, if applicable, to determine changes.
Watch for crackles, cough, tachypnea, and edema, which may indicate impending left-sided heart failure. Carefully monitor daily weight, intake and output, respiratory rate, enzyme levels, ECG readings, and blood pressure. Auscultate for adventitious breath sounds periodically (patients on bed rest frequently have atelectatic crackles, which may disappear after coughing) and for S3 or S4 gallops.
Organize patient care and activities to maximize periods of uninterrupted rest.
 TEACHING THE PATIENT WITH ACS
TEACHING THE PATIENT WITH ACS
To promote compliance with the prescribed medication regimen and other treatment measures, thoroughly explain dosages and therapy. Inform the patient of the drug’s adverse reactions, and advise him to watch for and report signs and symptoms of toxicity (for example, anorexia, nausea, vomiting, mental depression, vertigo, blurred vision, and yellow vision, if the patient is receiving a cardiac glycoside).
Review dietary restrictions with the patient. If he must follow a low-sodium, low-fat, or low-cholesterol diet, provide a list of foods to avoid. Ask the dietitian to speak to the patient and his family.
Encourage the patient to participate in a cardiac rehabilitation exercise program. The practitioner and the exercise physiologist should determine the level of exercise and then discuss it with the patient and secure his agreement to a stepped-care program.
Counsel the patient to resume sexual activity progressively. He may need to take nitroglycerin before sexual intercourse to prevent chest pain from the increased activity.
Advise the patient about appropriate responses to new or recurrent symptoms.
Advise the patient to report typical or atypical chest pain. Post-myocardial infarction (MI) syndrome may develop, producing chest pain that must be differentiated from a recurrent MI, pulmonary infarction, and heart failure.
Stress the need to stop smoking. If necessary, refer the patient to a support group.
If the patient has a Holter monitor in place, explain its purpose and use.
Review follow-up procedures, such as office visits and treadmill testing, with the patient.
Ask the dietary department to provide a clear liquid diet until nausea subsides. A low-cholesterol, low-sodium diet, without caffeine, may be ordered. (See Teaching the patient with ACS.)
Provide a stool softener to prevent straining during defecation, which causes vagal stimulation and may slow heart rate.
Allow the patient to use a bedside commode, and provide as much privacy as possible.
Assist with range-of-motion exercises. If the patient is immobilized by a severe MI, turn him often. Antiembolism stockings help prevent venostasis and thrombophlebitis.
Initiate a cardiac rehabilitation program, which typically includes education regarding heart disease, exercise, and emotional support for the patient and his family.
Provide emotional support, and help reduce stress and anxiety; administer a tranquilizer if needed.
If the patient has undergone PTCA, sheath care is necessary. Keep the sheath line open with a heparin drip. Observe the patient for generalized and site bleeding. Keep the leg with the sheath insertion site immobile. Maintain strict bed rest. Check peripheral pulses in the affected leg frequently. Provide an analgesic for back pain, if needed.
After thrombolytic therapy, administer continuous heparin. Monitor the partial thromboplastin time every 6 hours, and monitor the patient for evidence of bleeding.
Monitor ECG rhythm strips for reperfusion arrhythmias, and treat them according to facility protocol. If the artery reoccludes, the patient experiences the same symptoms as before. If this occurs, prepare the patient for return to the cardiac catheterization laboratory.
low-density lipoprotein (LDL) receptor—a protein that removes LDL from the bloodstream; a mutation in this gene is responsible for familial hypercholesterolemia
apolipoprotein E—mutations in this gene, commonly called apo E, also affects blood levels of LDL
apolipoprotein B-100—commonly called apo B-11, it’s a component of LDL; mutations of the gene cause LDL to stay in the blood longer than normal, leading to high LDL levels
apolipoprotein A—a glycoprotein that combines with LDL to form a particle called Lp(a); it appears as a part of plaque on blood vessels
MTHFR—an enzyme that clears homocysteine from the blood; mutations in MTHFR genes may cause high homocysteine levels
cystathionine B-synthase—also known as CBS, it’s another enzyme involved in homocysteine metabolism; CBS mutations cause a condition known as homocystinuria (homocysteine levels are so high that homocysteine can be detected in urine).
age—Atherosclerosis usually occurs after age 40.
sex—Men are eight times more susceptible than premenopausal women.
heredity—A positive family history of CAD increases the risk. (See Exploring the genetic link to CAD.)
race—White men are more susceptible than nonwhite men, and nonwhite women are more susceptible than white women.
high blood pressure—Systolic blood pressure that’s higher than 160 mm Hg or diastolic blood pressure that’s higher than 95 mm Hg increases the risk.
high cholesterol levels—Increased low-density lipoprotein and decreased high-density lipoprotein levels substantially heighten the risk.
smoking—Cigarette smokers are twice as likely to have an MI and four times as likely to experience sudden death. The risk dramatically drops within 1 year after smoking ceases.
obesity—Added weight increases the risk of diabetes mellitus, hypertension, and elevated cholesterol levels.
physical inactivity—Regular exercise reduces the risk.
stress—Added stress or a type A personality increases the risk.
diabetes mellitus—This disorder raises the risk, especially in women.
other modifiable factors—Increased fibrinogen and uric acid levels, elevated hematocrit, reduced vital capacity; high resting heart rate, thyrotoxicosis, and use of a hormonal contraceptive heightens the risk.
Arrhythmias
Ischemic cardiomyopathy
MI
The classic symptom of CAD is angina, the direct result of inadequate oxygen flow to the myocardium. The patient usually describes it as a burning, squeezing, or crushing tightness in the substernal or precordial chest that may radiate to the left arm, neck, jaw, or shoulder blade. Typically, the patient clenches his fist over his chest or rubs his left arm when describing the pain. Nausea, vomiting, fainting, sweating, and cool extremities may accompany the tightness.
may also develop during sleep from which symptoms awaken the patient. (See Types of angina.)
altered influx of calcium across the cell membrane
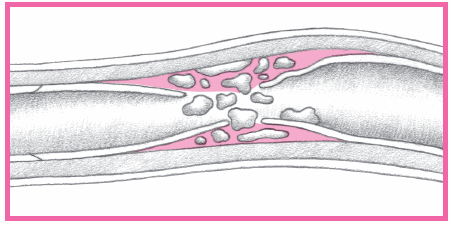
intimal hemorrhage into the medial layer of the blood vessel
hyperventilation
an elevated catecholamine level
fatty buildup in the lumen.
Stable angina—pain is predictable in frequency and duration and is relieved by rest and nitroglycerin.
Unstable angina—pain increases in frequency and duration and is more easily induced; it indicates a worsening of coronary artery disease that may progress to a myocardial infarction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree