Gastrointestinal bleeding (GIB) complicating percutaneous coronary intervention (PCI) results in high mortality, but clinical factors associated with and long-term outcomes of GIB are poorly understood. We sought to examine clinical and procedural factors associated with GIB complicating PCI. We also examined the impact of GIB on 30-day mortality and 1-year major adverse cardiac events (MACEs). Patients undergoing PCI from January 2000 to January 2010 were retrospectively analyzed for the occurrence of in-hospital GIB. Multivariable logistic regression and Cox proportional hazards regression were used to identify predictors of in-hospital GIB and 30-day mortality. Landmark analysis of patients surviving to hospital discharge was performed to assess the impact of GIB on 1-year MACEs. Of 20,621 patients who underwent PCI, 147 (0.72%) who developed in-hospital GIB were identified. Variables associated with increased risk of GIB included older age, shock, acute myocardial infarction, chronic renal insufficiency, lower baseline hematocrit, and glycoprotein IIb/IIIa inhibitors; bivalirudin decreased the risk. Unadjusted 30-day mortality rate of patients with GIB was 20.5% compared to 2.4% of patients without GIB. After multivariable adjustment, GIB and shock (and an interaction between the 2) were the most important correlates of 30-day mortality. In the population surviving to discharge, however, GIB was not associated with adjusted mortality or MACEs. In conclusion, GIB complicating PCI has a dramatic impact on 30-day mortality, and bivalirudin was associated with lower rates of GIB.
Antiplatelet and antithrombotic medications for treatment of patients with an acute coronary syndrome or undergoing percutaneous coronary intervention (PCI) decrease ischemic events and mortality, but they also confer an inherent risk of bleeding. Indeed, the risk of major bleeding in high-risk populations may approach 5%. Furthermore, major bleeding complicating an acute coronary syndrome is associated with up to a fivefold increase in death. Gastrointestinal bleeding (GIB) is an uncommon complication of PCI, with an incidence of 1.2% to 2.3% reported. It is of particular concern, however, because of the dramatic increase in mortality that accompanies GIB. This is not only apparent in unselected patients; the effect is especially pronounced in patients with an acute coronary syndrome or undergoing PCI. Risk factors for GIB complicating PCI and ischemic outcomes in such patients remain poorly studied. In particular, the individual effect of the many currently available adjunctive pharmacologic therapies on likelihood of GIB remains obscure. Furthermore, GIB may prompt premature cessation of dual antiplatelet therapy, but long-term ischemic outcomes in this setting are not well studied. We therefore sought to examine clinical and procedural factors associated with in-hospital GIB complicating PCI and to assess the impact of such GIB on short-term mortality and long-term ischemic outcomes.
Methods
Clinical, procedural, and follow-up data for patients undergoing PCI at a single center were prospectively entered and retrospectively analyzed. Indications for PCI included stable angina, unstable angina, and acute myocardial infarction. This study consisted of patients undergoing PCI with bare metal or drug-eluting stents from January 2000 to January 2010.
All patients received aspirin 325 mg and clopidogrel 300 to 600 mg (at the operator’s discretion) before the procedure. Anticoagulation regimens were chosen at the operator’s discretion and included unfractionated heparin targeted to achieve an activated clotting time <200 to 300 seconds, with or without a glycoprotein IIb/IIIa inhibitor, or bivalirudin 0.75 mg/kg followed by an infusion of 1.75 mg/kg/hour for the duration of the procedure. Patients presenting for rescue PCI after thrombolytics were included. After the procedure, aspirin 325 mg was prescribed indefinitely and clopidogrel 75 mg was prescribed for a minimum of 1 month in patients receiving bare metal stents and 6 months in patients receiving drug-eluting stents.
The institutional review board at Washington Hospital Center and MedStar Health Research Institute (Washington, DC) approved this study. A dedicated data coordinating center performed all data management and analyses. Prespecified clinical and laboratory data during hospitalization periods were obtained from hospital charts reviewed by independent research personnel blinded to objectives of the study. All patients routinely underwent 12-lead electrocardiography before and after PCI to detect procedure-related ischemic changes and/or presence of new pathologic Q waves. Blood samples at 6 and 24 hours before and after PCI were drawn to assess the creatine kinase-MB biomarker. If creatine kinase-MB was increased above the reference range (4 mg/dl), measurements were repeated every 8 hours until it returned to below the reference range. Clinical follow-up at 30 days and 1 year was conducted by telephone contact or office visits. Occurrence of major late clinical events was recorded, including death (all-cause), Q-wave myocardial infarction, target vessel revascularization, and stent thrombosis.
GIB was defined as clinical (coffee-ground emesis, melena, or hematochezia) or endoscopic evidence of an actively bleeding upper or lower site. Major adverse cardiac events (MACEs) were defined as a composite of death from all causes, Q-wave myocardial infarction, and target vessel revascularization. Q-wave myocardial infarction was defined as an appearance of new pathologic Q waves in the coronary distribution of the treated artery with an increase of creatine kinase-MB to ≥2 times the reference value. Target vessel revascularization was defined as revascularization occurring in any area along the previously treated vessel. Acute myocardial infarction was defined as presentation with non–ST-segment elevation myocardial infarction or ST-segment elevation myocardial infarction. Receipt of heparin was defined as >2,000 U unfractionated heparin or any low-molecular-weight heparin before or during PCI. Patients receiving thrombolytics included those with rescue PCI and those receiving intracoronary lytics.
Continuous variables are presented as mean ± SD; categorical variables are presented as percentages. For variables with missing data, percentages were calculated using the number of patients with data present for that variable. Differences in continuous variables between groups were compared using Student’s t test. Categorical variables were compared using chi-square test or Fisher’s exact test as appropriate. Thirty-day and 1-year outcomes were compared using the log-rank test and are presented as Kaplan-Meier percentages. A p value <0.05 was considered statistically significant.
To test the independent effect of clinical and procedural characteristics on occurrence of in-hospital GIB, we first constructed a multivariable logistic regression model. This model included shock on presentation, acute myocardial infarction, age, male gender, current smoking, baseline hematocrit, chronic renal insufficiency, diabetes mellitus, and receipt of thrombolytic, heparin, glycoprotein IIb/IIIa inhibitor, and bivalirudin. To test the independent effect of in-hospital GIB on mortality at 30 days, we constructed a multivariable Cox regression model. This model included in-hospital GIB, shock, an interaction term between GIB and shock, acute myocardial infarction, male gender, age, current smoking, chronic renal insufficiency, diabetes mellitus, hypertension, previous PCI, previous coronary artery bypass grafting, congestive heart failure, and peripheral vascular disease. Because the interaction term between GIB and shock was highly significant, we constructed a categorical variable with the following groups: GIB and shock, GIB and no shock, no GIB and shock; no GIB and no shock was the reference group. The multivariable Cox model was then adjusted accordingly. We also performed a landmark analysis of MACEs at 1 year in patients who survived to hospital discharge, with essentially the same covariables as the multivariable Cox model for mortality at 30 days. We added receipt of ≥1 drug-eluting stent, however, to this model, because length of dual antiplatelet therapy may be a significant confounding variable in an analysis of bleeding events. Furthermore, the interaction term between GIB and shock was no longer significant and thus excluded from the model. We selected covariables for each model based on significant univariable p values and overall clinical relevance. Only patients with data present for all covariables are included in each model. Covariables in each model are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
This study included 147 patients (0.72%) with in-hospital GIB and 20,474 without in-hospital GIB ( Table 1 ). Twenty-five patients (17.0%) with GIB showed a hematocrit decrease ≥15% and 106 (74.6%) required a blood product transfusion ≥1 U compared to 329 patients (1.6%) and 863 patients (4.4%) without GIB (p <0.001).
| Variable | In-Hospital GIB | p Value | |
|---|---|---|---|
| Yes (n = 147) | No (n = 20,474) | ||
| Age (years) | 70.3 ± 13.0 | 64.5 ± 12.6 | <0.001 |
| Men | 76 (52.1%) | 13,297 (65.5%) | <0.001 |
| Caucasian | 90 (61.2%) | 13,416 (66.0%) | 0.22 |
| African-American | 42 (28.6%) | 4,908 (24.1%) | 0.21 |
| Asian | 8 (5.4%) | 823 (4.0%) | 0.39 |
| Hispanic | 0 | 240 (1.2%) | 0.42 |
| ST-segment elevation myocardial infarction | 19 (22.4%) | 1,793 (12.6%) | 0.01 |
| Non– ST-segment elevation myocardial infarction | 36 (34.0%) | 1,715 (11.7%) | <0.001 |
| Shock on presentation | 30 (21.0%) | 612 (3.1%) | <0.001 |
| Previous coronary artery bypass surgery | 33 (22.9%) | 4,247 (21.0%) | 0.58 |
| Previous percutaneous coronary intervention | 30 (24.6%) | 5,183 (27.2%) | 0.52 |
| Congestive heart failure | 51 (35.9%) | 3,135 (16.1%) | <0.001 |
| Diabetes mellitus | 58 (39.7%) | 7,200 (35.7%) | 0.31 |
| Systemic hypertension ⁎ | 122 (83.6%) | 17,079 (84.3%) | 0.80 |
| Chronic renal insufficiency | 54 (37.2%) | 2,754 (13.7%) | <0.001 |
| Peripheral vascular disease | 51 (36.7%) | 3,293 (16.4%) | <0.001 |
| Current smoker | 30 (20.4%) | 4,529 (22.3%) | 0.59 |
| Baseline hematocrit (%) | 35.2 ± 7.6 | 39.4 ± 7.2 | <0.001 |
| Baseline platelet count (×10 9 /L) | 254 ± 102 | 228 ± 103 | 0.003 |
| Heparin | 90 (61.2%) | 9,315 (45.8%) | <0.001 |
| Thrombolytics | 10 (7.4%) | 839 (4.3%) | 0.09 |
| Glycoprotein IIb/IIIa inhibitor | 46 (31.9%) | 3,424 (17.0%) | <0.001 |
| Bivalirudin | 64 (43.5%) | 12,835 (63.1%) | <0.001 |
⁎ History of hypertension diagnosed and/or treated with medication or currently being treated with diet and/or medication by a physician.
Patients with GIB complicating PCI were more likely to be older, be women, present with shock or acute myocardial infarction (ST elevation and non–ST elevation), and have a history of congestive heart failure, chronic renal insufficiency, or peripheral vascular disease compared to patients without GIB. Baseline hematocrit was also lower in patients with GIB (35.2 ± 7.6%) compared to patients without GIB (39.4 ± 7.2%, p <0.001); baseline platelet count, however, was higher in patients with GIB (254 ± 102 × 10 9 /L) compared to patients without GIB (228 ± 103 × 10 9 /L, p <0.001). The proportion of patients on warfarin in each group was similar (7.1% overall), although this information was available for only about 70% of patients in each group. Patients with GIB were more likely to receive heparin and glycoprotein IIb/IIIa inhibitors and less likely to receive bivalirudin than patients without GIB. Patients with GIB and receiving heparin were no more likely to have an activated clotting time >300 seconds than were patients without GIB (27.8% overall). Patients with GIB were, however, less likely to receive ≥1 drug-eluting stent (42.6% vs 68.2%, p <0.001).
Covariables significantly associated with GIB ( Table 2 ) included increasing age (odds ratio [OR] per 10 years 1.34, 95% CI 1.13 to 1.58, p = 0.001), shock (OR 4.58, 95% CI 2.74 to 7.67, p <0.001), acute myocardial infarction (OR 2.43, 95% CI 1.58 to 3.74, p <0.001), chronic renal insufficiency (OR 2.49, 95% CI 1.66 to 3.73, p <0.001), and lower baseline hematocrit (OR per 5% increase 0.64, 95% CI 0.56 to 0.72, p <0.001). Patients receiving a glycoprotein IIb/IIIa inhibitor were more likely to develop GIB (OR 1.85, 95% CI 1.17 to 2.91, p = 0.008), whereas those receiving bivalirudin were less likely (OR 0.58, 95% CI 0.36 to 0.94, p = 0.03). Neither thrombolytics nor heparin was associated with GIB. Results from a similar analysis comparing patients with GIB to only patients without a hematocrit decrease ≥15% or hematoma ≥4 cm did not change the results significantly.
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Age (per 10 years) | 1.34 | 1.13–1.58 | 0.001 |
| Shock | 4.58 | 2.74–7.67 | <0.001 |
| Acute myocardial infarction ⁎ | 2.43 | 1.58–3.74 | <0.001 |
| Chronic renal insufficiency | 2.49 | 1.66–3.73 | <0.001 |
| Baseline hematocrit (per 5%) | 0.64 | 0.56–0.72 | <0.001 |
| Glycoprotein IIb/IIIa inhibitor | 1.85 | 1.17–2.91 | 0.008 |
| Bivalirudin | 0.58 | 0.36–0.94 | 0.03 |
| Heparin | 0.79 | 0.50–1.25 | 0.32 |
| Thrombolytics | 0.74 | 0.36–1.55 | 0.43 |
⁎ Presenting with ST-segment elevation or non–ST-segment elevation myocardial infarction.
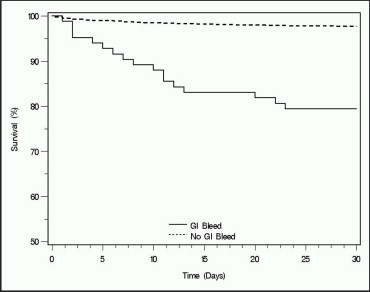
In-hospital GIB was associated with a remarkably high mortality rate of 20.5% at 30 days compared to 2.4% in patients without GIB (p <0.001 for log-rank test; Figure 1 ). Rates of other cardiovascular outcomes, however, including target vessel revascularization (1.0% overall), Q-wave myocardial infarction (0.1%), and stent thrombosis (0.5%), were similar between patients with and without GIB.