Recent studies have suggested that percutaneous coronary intervention (PCI) in patients with unprotected left main coronary artery (LMCA) disease renders outcomes comparable to those from coronary artery bypass grafting (CABG). It is necessary to stratify individual patient risk and select the optimal revascularization strategy. We compared the clinical outcomes of patients with unprotected LMCA disease who had undergone PCI with drug-eluting stents or CABG. We identified 462 patients who were treated from January 2003 to December 2006 for unprotected LMCA or LMCA-equivalent disease: 257 had undergone CABG and 205 had undergone PCI with drug-eluting stents. Analyses using propensity scores were performed to minimize the selection bias in the present observational study. After a median follow-up of 33.5 months, no significant difference was found between the CABG and PCI groups in the risk of death (12.1% vs 14.1%, respectively; p = 0.428) or the risk of a composite of death, myocardial infarction, or cerebrovascular accident (17.5% vs 20.0%, respectively; p = 0.434). The rate of major adverse cardiac and cerebrovascular events was significantly lower in the CABG group than in the PCI group (21.8% vs 35.1%, respectively; p = 0.001); the difference was mainly driven by a decrease in the rate of repeat revascularizations (5.1% vs 22.4%; p <0.001). The analyses after propensity score adjustment and matching corroborated the crude group results. In conclusion, PCI with drug-eluting stents showed a safety profile comparable to that of CABG in patients with unprotected LMCA disease. However, the risk of repeat revascularization was significantly greater in the PCI group.
For several decades, coronary artery bypass grafting (CABG) has been the treatment of choice for patients with significant unprotected left main coronary artery (LMCA) stenosis. Recent improvements in percutaneous coronary intervention (PCI) techniques and the introduction of drug-eluting stents have indicated the need for a paradigm shift in the treatment of LMCA lesions. The support for such a shift has come from the increasing amount of data suggesting fair outcomes after PCI for unprotected LMCA disease. PCI has shown safety outcomes comparable to those of CABG; however, PCI has been associated with a greater rate of repeat revascularization. Although these results might urge clinicians to change their current practice patterns, a paucity of data is available to confirm the comparability of the 2 treatment strategies. Also, no stratification of the treatment benefits and risks has been developed that would help guide decisions on optimal treatment options for patients with LMCA disease. Using the Seoul National University Main and Bundang Hospital Left Main Registries, we compared the long-term (3-year) outcomes of patients with unprotected LMCA disease who had undergone PCI with a drug-eluting stent or CABG. We evaluated the clinical and angiographic prognostic factors and identified the factors that could guide in the selection of an optimal revascularization strategy.
Methods
The present study was an analysis of a 2-center registry of unprotected LMCA disease from the Seoul National University Hospital and Seoul National University Bundang Hospital (Seoul, Korea). From January 2003 to December 2006, 462 patients underwent revascularization treatment. Of these 462 patients, 257 underwent CABG and 205 underwent PCI with a drug-eluting stent for de novo lesions in unprotected LMCA or LMCA-equivalent disease. LMCA disease was defined as >50% LMCA stenosis and LMCA-equivalent disease as severe (≥70%) proximal left anterior descending and proximal left circumflex stenoses. It was an “all-comer analysis” that included patients who presented with acute myocardial infarction or cardiogenic shock and those who underwent emergency procedures. Before determining the revascularization modality, the treatment strategies were carefully discussed with the patient, interventional cardiologist, cardiac surgeon, and attending physician. The local institutional review board approved the study protocol, which was in accordance with the Declaration of Helsinki.
The interventional and operational procedures were performed according to current standard techniques. CABG was performed either “on pump” using cardioplegics or “off pump.” When possible, the internal thoracic arteries were harvested, and the left anterior descending artery was revascularized. If the use of bilateral internal thoracic arteries as in situ or Y grafts did not achieve complete revascularization, the right gastroepiploic artery, radial artery, or saphenous vein was used for additional revascularization. All patients undergoing PCI were premedicated with a loading dose of aspirin (300 mg) and clopidogrel (300 to 600 mg). Unfractionated heparin was administered either before or during PCI to achieve an activated clotting time of ≥300 seconds. The type of drug-eluting stent to be implanted was decided by the operator. Either the radial or femoral artery was used for vascular access; and the use of glycoprotein IIb/IIIa inhibitors, predilation devices, stenting techniques, and intravascular ultrasound guidance was at the operators’ discretions. After PCI, lifelong aspirin and dual antiplatelet therapy for ≥6 months were recommended to all patients.
Clinical follow-up examinations after PCI or CABG was recommended at 1, 3, 6, and 12 months and then every 6 months thereafter. The clinical data were collected for ≤3 years after the index procedure. Although routine angiographic follow-up was recommended at 6 months after PCI, it was left to the surgeon’s discretion after CABG. The clinical, angiographic, procedural, and outcome data were collected by independent nurses and researchers who had not participated in the treatment of the study patients. The subjects who had not adhered to the recommended follow-up processes were interviewed by telephone at regular 6-month intervals. To validate complete follow-up data, vital status information was obtained from the Korea National Statistical Office using a unique personal identification number.
The study population was followed for the occurrence of clinical events, including death, myocardial infarction, cerebrovascular accident, and target vessel revascularization (TVR). Death was classified as from either cardiac or noncardiac causes, according to the Academic Research Consortium definition. All deaths were considered cardiac in origin unless a noncardiac origin had definitely been documented. Myocardial infarction was defined according to the recommendations of the European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force. TVR was defined as the repeat intervention (surgical or percutaneous) of any segment of the treated vessel, including the left main, left anterior descending, and left circumflex arteries. cerebrovascular accident, including both ischemic and hemorrhagic stroke, was confirmed by a neurologist from the imaging study findings. Major adverse cardiac and cerebrovascular events were defined as a composite of death, myocardial infarction, cerebrovascular accident, or TVR. The occurrence of “definite” and “probable” stent thrombosis (ST), according to the Academic Research Consortium definition, or of symptomatic graft occlusion after CABG was recorded. ST was stratified relative to the timing of the events as acute (0 to 24 hours), subacute (>24 hours to 30 days), late (31 days to 1 year), and very late (>1 year).
The baseline data are presented as the frequency or mean ± SD. Continuous variables were compared using the Student t tests, and categorical variables were compared using the chi-square tests. Because of the nonrandomized observational nature of the study, propensity score analyses were performed to minimize any selection bias. In brief, propensity to each treatment strategy was scored using multivariate logistic regression analysis for every patient with 27 variables, including demographic characteristics, co-existing medical conditions, and laboratory and angiographic findings. The individual propensity score was incorporated into the Cox regression model as a covariate to calculate the propensity-adjusted hazard ratio. In addition, after patients were matched according to the propensity score in a 1:1 ratio, the risk of each outcome was compared to the matched cohort. A 2-sided p value of <0.05 was considered significant for all tests. All statistical analyses were performed using Stata/MP, version 10.0 for Windows (StataCorp, College Station, Texas).
Results
Table 1 lists a comparison of the baseline characteristics of the 2 treatment groups. The patients treated with CABG were more likely to have peripheral artery disease and a familial history of coronary artery disease and had a greater mean EuroSCORE. Angiographically, coronary artery disease was more extensive and right coronary artery and distal bifurcation involvement were more common in the CABG group. Most of the CABG cases used internal thoracic arteries and anastomosized the left anterior descending artery ( Table 2 ). CABG was performed off pump in 71.6% of patients. Most PCIs were performed with a first-generation drug-eluting stent (sirolimus-eluting or paclitaxel-eluting stent); however, zotarolimus-eluting stents were used in 7 patients. Ostial or shaft lesions were generally treated with a single stent. In lesions involving the distal bifurcation, a variety of techniques were applied after consideration of angiographic factors such as the presence of left circumflex ostial disease and reference vessel size. Of the lesions involving distal bifurcation, “final kissing ballooning” was performed in 38.5% of the cases.
| Variable | CABG (n = 257) | PCI (n = 205) | p Value |
|---|---|---|---|
| Men | 190 (73.9%) | 144 (70.2%) | 0.379 |
| Age (years) | 65.7 ± 10.0 | 64.2 ± 11.5 | 0.121 |
| Diabetes mellitus | 112 (43.6%) | 77 (37.6%) | 0.191 |
| Hypertension | 173 (67.3%) | 130 (63.4%) | 0.381 |
| Current smoking | 127 (49.4%) | 89 (43.4%) | 0.199 |
| Body mass index (kg/m 2 ) | 24.0 ± 2.8 | 23.8 ± 3.1 | 0.501 |
| History of cerebrovascular disease | 27 (10.5%) | 30 (14.6%) | 0.180 |
| Peripheral artery disease | 87 (33.9%) | 20 (9.8%) | <0.001 |
| Familial history of coronary artery disease | 34 (13.2%) | 15 (7.3%) | 0.040 |
| Dyslipidemia | 153 (59.5%) | 112 (54.6%) | 0.290 |
| Chronic kidney disease | 28 (10.9%) | 21 (10.2%) | 0.821 |
| End-stage renal disease | 7 (2.7%) | 10 (4.9%) | 0.222 |
| History of percutaneous coronary intervention | 9 (3.5%) | 46 (22.4%) | <0.001 |
| Clinical diagnosis | <0.001 | ||
| Stable angina pectoris | 73 (28.4%) | 76 (37.1%) | |
| Unstable angina pectoris | 145 (56.4%) | 77 (37.6%) | |
| Non–stent thrombosis-elevation myocardial infarction | 21 (8.2%) | 16 (7.8%) | |
| Stent thrombosis-elevation myocardial infarction | 10 (3.9%) | 27 (13.2%) | |
| Old myocardial infarction | 8 (3.1%) | 9 (4.4%) | |
| Shock at admission | 10 (3.9%) | 7 (3.4%) | 0.787 |
| Mean EuroSCORE | 5.6 ± 3.8 | 4.2 ± 3.9 | <0.001 |
| EuroSCORE ≥ 6 | 116 (45.1%) | 49 (23.9%) | <0.001 |
| EuroSCORE ≥13 | 15 (5.8%) | 10 (4.9%) | 0.651 |
| Follow-up angiography | 148 (57.8%) | 165 (80.5%) | <0.001 |
| Follow-up duration (months) | 0.115 | ||
| Median | 35.0 | 31.6 | |
| Interquartile range | 24.5–36.0 | 22.1–36.0 | |
| Serum creatinine (mg/dl) | 1.3 ± 1.1 | 1.4 ± 1.3 | 0.628 |
| Total cholesterol (mg/dl) | 172 ± 44 | 167 ± 43 | 0.264 |
| Low-density lipoprotein cholesterol (mg/dl) | 100 ± 38 | 96 ± 37 | 0.272 |
| High-density lipoprotein cholesterol (mg/dl) | 43 ± 13 | 43 ± 11 | 0.887 |
| Triglycerides (mg/dl) | 135 ± 83 | 126 ± 67 | 0.243 |
| Left ventricular ejection fraction (%) | 55.4 ± 12.5 | 55.5 ± 11.6 | 0.967 |
| Extent of involved vessel | <0.001 | ||
| Left main disease alone | 15 (5.8%) | 30 (14.6%) | |
| Left main plus 1-vessel disease | 23 (8.9%) | 65 (31.7%) | |
| Left main plus 2-vessel disease | 64 (24.9%) | 42 (20.5%) | |
| Left main plus 3-vessel disease | 155 (60.3%) | 68 (33.2%) | |
| Right coronary artery involvement | 190 (73.9%) | 112 (54.6%) | <0.001 |
| Disease location | <0.001 | ||
| Ostium | 35 (13.6%) | 32 (15.6%) | |
| Shaft | 41 (16.0%) | 11 (5.4%) | |
| Distal bifurcation | 176 (68.5%) | 123 (60.0%) | |
| Diffuse | 3 (1.2%) | 39 (19.0%) | |
| Bifurcation type by Medina classification | 185 | 160 | 0.326 |
| (1,1,1) | 77 (41.6%) | 65 (40.6%) | |
| (1,1,0) | 54 (29.2%) | 43 (26.9%) | |
| (1,0,1) | 23 (12.4%) | 15 (9.4%) | |
| (0,1,1) | 14 (7.6%) | 23 (14.4%) | |
| (1,0,0) | 17 (9.2%) | 14 (8.8%) | |
| Glycoprotein IIb/IIIa inhibitors | 2 (0.8%) | 17 (8.3%) | <0.001 |
| Emergency procedure | 26 (10.1%) | 28 (13.7%) | 0.239 |
| Intra-aortic balloon counterpulsation | 74 (28.8%) | 27 (13.2%) | <0.001 |
| Medications at discharge | |||
| Angiotension-converting enzyme inhibitor | 40 (16.0%) | 68 (34.5%) | <0.001 |
| Angiotension receptor blocker | 55 (22.0%) | 39 (19.8%) | 0.570 |
| β Blockers | 90 (36.0%) | 120 (60.9%) | <0.001 |
| Calcium channel blockers | 119 (47.6%) | 50 (25.4%) | <0.001 |
| Statins | 133 (53.2%) | 124 (62.9%) | 0.039 |
| Duration of dual antiplatelet therapy (months) | |||
| Median | 15.9 | — | — |
| Interquartile range | 11.0–26.4 |
| Procedural Data | Value |
|---|---|
| Coronary artery bypass grafting (n = 257) | |
| Number of grafts | 3.2 ± 1.0 |
| Use of conduits | |
| Left internal thoracic artery | 242 (94.2%) |
| Right internal thoracic artery | 115 (44.7%) |
| Bilateral internal thoracic artery | 108 (42.0%) |
| Saphenous venous graft | 76 (29.6%) |
| Gastroepiploic artery | 126 (49.0%) |
| Radial artery | 35 (13.6%) |
| Graft to left anterior descending artery | 248 (96.5%) |
| Off-pump coronary artery bypass grafting | 184 (71.6%) |
| Percutaneous coronary intervention (n = 205) | |
| Procedural success | 201 (98.0%) |
| Implanted stents (n) | |
| 1 | 134 (65.4%) |
| 2 | 67 (32.7%) |
| 3 | 4 (2.0%) |
| Implanted stent type | |
| Sirolimus-eluting stent (Cypher) | 144 (70.2%) |
| Paclitaxel-eluting stent (Taxus) | 54 (26.3%) |
| Zotarolimus-eluting stent (Endeavor) | 7 (3.4%) |
| Technique | |
| Single stenting only | 37 (18.0%) |
| Crossover | 98 (47.8%) |
| Kissing stenting | 29 (14.1%) |
| Crush technique | 19 (9.3%) |
| T/modified T stenting | 22 (10.7%) |
| Maximum stent diameter (mm) | 3.3 ± 0.3 |
| Total stent length (mm) | 48.0 ± 9.6 |
| Final kissing stenting | 79 (38.5%) |
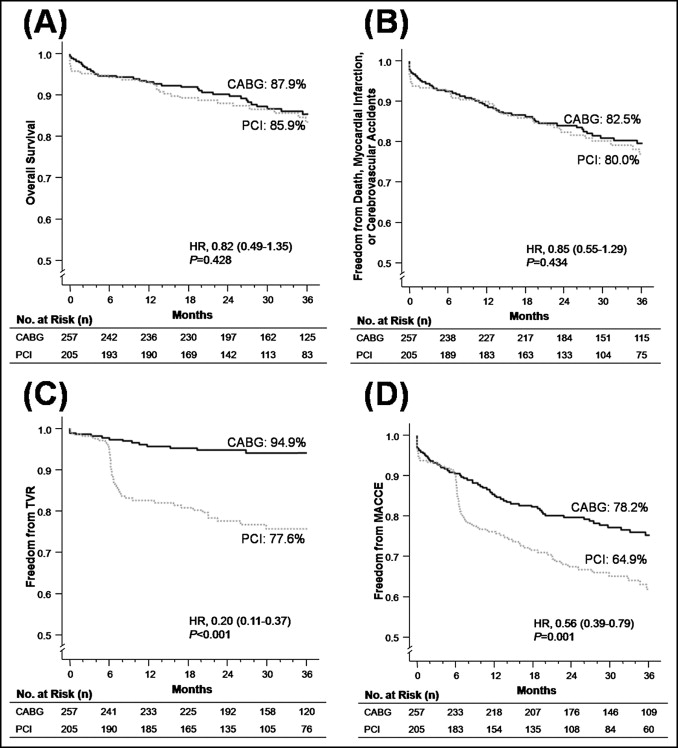
Figure 1 shows the clinical outcomes of the crude study population. After a median follow-up of 33.5 months (interquartile range 22.8 to 36.0), the mortality rate was 12.1% in the CABG group and 14.1% in the PCI group, and the overall incidence of the composite of death, myocardial infarction, and cerebrovascular accident was 17.5% and 20.0%, respectively. The rate of TVR in the CABG group was significantly lower than that in the PCI group (5.1% vs 22.4%; p <0.001; Table 3 ), resulting in a significantly lower rate of major adverse cardiac and cerebrovascular events in the CABG group (21.8% vs 35.1%; p = 0.001). Moreover, the rate of target lesion revascularization (i.e., excluding non-target lesion TVR) after PCI was 13.7%, and was still greater than the TVR rate after CABG (5.1%, p = 0.001). The data for each clinical outcome event at each follow-up time point are listed in Table 3 . Although the mortality rate in the PCI group was significantly greater at the initial 30 days, the difference was no longer present at 6 months. Although statistically insignificant, the cerebrovascular accident rates in all follow-up periods were consistently greater in the CABG group. The rate of TVR in the 2 groups started to diverge at 6 months of follow-up.
| Variable | CABG (n = 257) | PCI (n = 205) | OR (95% CI) | p Value |
|---|---|---|---|---|
| 30-Day outcomes | ||||
| Death from any cause | 3 (1.2%) | 9 (4.4%) | 0.26 (0.07–0.96) | 0.044 |
| Cardiac death | 3 (1.2%) | 9 (4.4%) | 0.26 (0.07–0.96) | 0.044 |
| Myocardial infarction | 1 (0.4%) | 3 (1.5%) | 0.26 (0.03–2.55) | 0.249 |
| Cerebrovascular accident | 5 (1.9%) | 2 (1.0%) | 2.01 (0.39–10.49) | 0.406 |
| Composite hard endpoint ⁎ | 8 (3.1%) | 13 (6.3%) | 0.48 (0.19–1.17) | 0.105 |
| Target vessel revascularization | 3 (1.2%) | 3 (1.5%) | 0.80 (0.16–3.98) | 0.780 |
| Major adverse cardiac and cerebrovascular events | 10 (3.9%) | 13 (6.3%) | 0.60 (0.26–1.39) | 0.233 |
| 6-Month outcomes | ||||
| Death from any cause | 13 (5.1%) | 11 (5.4%) | 0.94 (0.41–2.14) | 0.882 |
| Cardiac death | 8 (3.1%) | 10 (4.9%) | 0.63 (0.24–1.62) | 0.334 |
| Myocardial infarction | 1 (0.4%) | 3 (1.5%) | 0.26 (0.03–2.55) | 0.249 |
| Cerebrovascular accident | 8 (3.1%) | 2 (1.0%) | 3.26 (0.69–15.53) | 0.138 |
| Composite hard endpoint ⁎ | 18 (7.0%) | 15 (7.3%) | 0.95 (0.47–1.94) | 0.897 |
| Target vessel revascularization | 6 (2.3%) | 9 (4.4%) | 0.52 (0.18–1.49) | 0.223 |
| Major adverse cardiac and cerebrovascular events | 23 (8.9%) | 21 (10.2%) | 0.86 (0.46–1.61) | 0.638 |
| 1-Year outcomes | ||||
| Death from any cause | 16 (6.2%) | 14 (6.8%) | 0.91 (0.43–1.90) | 0.794 |
| Cardiac death | 10 (3.9%) | 12 (5.9%) | 0.65 (0.28–1.54) | 0.328 |
| Myocardial infarction | 3 (1.2%) | 5 (2.4%) | 0.49 (0.16–1.52) | 0.215 |
| Cerebrovascular accident | 8 (3.1%) | 2 (1.0%) | 3.26 (0.69–15.53) | 0.138 |
| Composite hard endpoint ⁎ | 23 (8.9%) | 20 (9.8%) | 0.74 (0.44–1.25) | 0.264 |
| Target vessel revascularization | 10 (3.9%) | 35 (17.1%) | 0.20 (0.11–0.39) | <0.001 |
| Major adverse cardiac and cerebrovascular events | 32 (12.5%) | 49 (23.9%) | 0.45 (0.28–0.74) | 0.002 |
| 2-Year outcomes | ||||
| Death from any cause | 23 (8.9%) | 24 (11.7%) | 0.74 (0.41–1.36) | 0.331 |
| Cardiac death | 15 (5.8%) | 17 (8.3%) | 0.69 (0.33–1.41) | 0.304 |
| Myocardial infarction | 5 (1.9%) | 8 (3.9%) | 0.49 (0.16–1.51) | 0.211 |
| Cerebrovascular accident | 8 (3.1%) | 2 (1.0%) | 3.24 (0.68–15.42) | 0.140 |
| Composite hard endpoint ⁎ | 32 (12.5%) | 33 (16.1%) | 0.79 (0.47–1.32) | 0.369 |
| Target vessel revascularization | 13 (5.1%) | 43 (21.0%) | 0.20 (0.10–0.38) | <0.001 |
| Major adverse cardiac and cerebrovascular events | 43 (16.7%) | 65 (31.7%) | 0.43 (0.28–0.67) | <0.001 |
| 3-Year outcomes | ||||
| Death from any cause | 31 (12.1%) | 29 (14.1%) | 0.83 (0.48–1.43) | 0.508 |
| Cardiac death | 18 (7.0%) | 21 (10.2%) | 0.66 (0.34–1.28) | 0.216 |
| Myocardial infarction | 7 (2.7%) | 10 (4.9%) | 0.55 (0.20–1.46) | 0.228 |
| Cerebrovascular accident | 16 (6.2%) | 6 (2.9%) | 2.20 (0.85–5.73) | 0.106 |
| Composite hard endpoint ⁎ | 45 (17.5%) | 41 (20.0%) | 0.85 (0.53–1.36) | 0.495 |
| Target vessel revascularization | 13 (5.1%) | 46 (22.4%) | 0.18 (0.10–0.35) | <0.001 |
| Major adverse cardiac and cerebrovascular events | 56 (21.8%) | 72 (35.1%) | 0.52 (0.34–0.78) | 0.002 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


