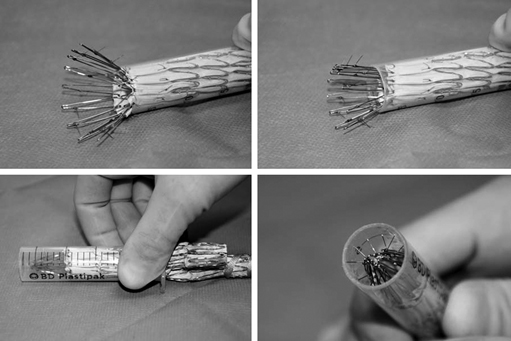
There are many helpful maneuvers to assist in successful removal of the aortic stent graft. The most commonly used is a traditional clamp-and-pull method. In stents with proximal barbs it is helpful to collapse the proximal stent. A technique we have used for removing suprarenal fixation devices with barbs involves collapsing the device into a 20-mL syringe (Figure 1). By cutting off the closed end of a syringe, the syringe cylinder can be then used as a type of short sheath to recapture and separate the proximal stent safely from the aortic wall. For endografts with nitinol stents, iced saline has been suggested to help reduce diameter and ease removal, but we have not found this effective in most circumstances.
Conversion to Open Surgical Treatment After Failed Endovascular Aortic Aneurysm Repair
Operative Techniques
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
