The advent of modern computed tomography (CT), including helical scanning technique and multidetector CT (MDCT) technology, has made noninvasive vascular imaging a clinical reality. Helical scanning combines a revolving gantry and detector element with a continuously moving table. Although initial setups suffered from differential through-plane resolution versus in-plane resolution, the continuous data acquisition resulted in a volumetric slab of data rather than a stack of interrupted slices. MDCT relies on the use of several detector-row elements placed adjacent to one another. These tiny elements individually facilitate higher through-plane resolution, but in aggregate they allow faster scanning through greater coverage per rotation.
State-of-the-art detector setups use 64 elements or more. As a result of these technological advances, modern MDCT scanners can acquire a complete volumetric data set in a single breath hold, providing submillimeter isotropic data resolution that can be reconstructed into any desired plane. Iodinated contrast material is required for vascular opacification, often introduced through large-bore peripheral intravenous access using a power injector device at 3 to 5 mL/sec. This rapid delivery of contrast material creates a relatively compact bolus that makes the abdominal aorta and renal arterial system opaque for a short duration. Because blood flow through the kidneys is brisk, accurate scan timing is critical to ensure peak arterial opacification with relatively little venous contamination.
Although several different timing mechanisms can be used in CT arteriography (CTA), the most reproducible method is an automated bolus-tracking technique that triggers acquisition from a threshold level of contrast delivery in a large vessel, such as the abdominal aorta. For bolus tracking in renal CTA, the suprarenal aorta is often used to monitor delivery of contrast. Once the imaging data have been acquired, review and diagnosis are often aided by postprocessing techniques, which can help delineate the three-dimensional (3-D) relationships of various structures. In particular, maximum-intensity-projection images and volume-rendered images are often generated for CTA studies to highlight and segment the arterial anatomy. Studies have shown similar sensitivities for arterial stenoses between the two techniques, though volume-rendered techniques have shown increased specificity. Whereas the 3-D reformatted imaging may be performed by a technologist, complete review of both the native axial imaging and postprocessed 3-D imaging is critical for establishing the correct diagnosis, because some features can be erroneously highlighted or overlooked based solely on postprocessed imaging.
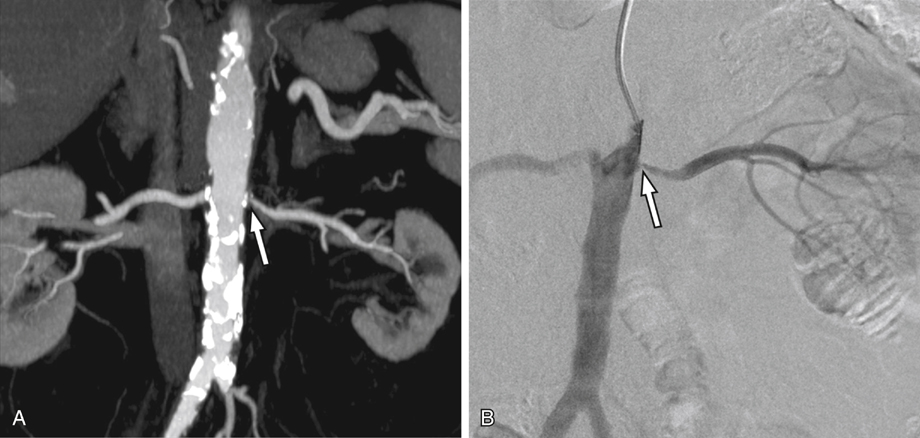
Evaluation of suspected renal arterial narrowing has become a major indication for renal CTA. Depiction of renal artery stenosis on CTA is relatively straightforward, particularly with reformatted imaging, where the arteries may be segmented or highlighted versus the background tissues.
Atherosclerotic disease and fibromuscular dysplasia can be well demonstrated on CTA (Figures 1 and 2). Narrowings may be graded qualitatively or quantitatively. Eccentric narrowings, such as the webs associated with fibromuscular dysplasia, are often best demonstrated in an orthogonal plane or using 3-D reformatted images. Initial reports on the use of CTA for investigation of suspected renal artery stenosis were strongly positive. Early reports showed a high accuracy for hemodynamically significant stenoses outside of the renal hilum, and subsequent studies demonstrated high sensitivity and accuracy in the evaluation of fibromuscular dysplasia. A large meta-analysis in 2001 that investigated CTA for use in suspected renovascular hypertension revealed a high level of diagnostic performance, including an area under the summary receiver operating characteristic (ROC) curve of 0.99.
Only gold members can continue reading.
Log In or
Register to continue
Related
![]()