5 Contrast-Induced Acute Kidney Injury and the Role of Chronic Kidney Disease in PCI
 Introduction
Introduction
Alterations of the kidney are commonly encountered in the interventional cardiology setting. Sudden changes are manifest during AKI that are sustained during procedures, while chronic decreased function is a predisposing risk factor. Contrast-induced AKI, also known as contrast-induced nephropathy, is a widely recognized complication of cardiac catheterization and PCI. A transient rise in serum creatinine levels, a common marker for the development of mild renal dysfunction, occurs in more than 15% of patients undergoing these procedures. Although many of these rises are unlikely to be clinically significant, even mild AKI after cardiac catheterization and PCI has been associated with longer hospital stays and greater inpatient costs as well as worse short- and long-term mortality. Contrast-induced AKI after cardiac catheterization and PCI is believed to be primarily caused by intraprocedural exposure to contrast agents, which are nephrotoxic in high-doses.1 The single most important risk factor that has been linked to the development of AKI after cardiac catheterization and PCI is the presence of preexisting CKD. Other clinical factors like diabetes mellitus and hemodynamic instability, which are also highly prevalent in this population, may also contribute to and exacerbate its clinical course. In a small proportion of patients, AKI may be related to renal atheroembolic disease from diffuse atherosclerosis of the aorta. In patients without CKD or other risk factors, the development of renal dysfunction after these procedures is rare. Importantly, recent diagnostic and therapeutic advances have improved our ability to identify those patients who are at highest risk for developing contrast-induced AKI and to minimize its occurrence. In this chapter we provide a summary of this data with an additional focus on patients with CKD, given its strong association with AKI as well as subsequent cardiovascular complications. We discuss the role of nonpharmacological and pharmacological strategies for reducing the likelihood that AKI will develop in high-risk patients. Finally, we briefly comment on two groups of patients who represent growing segments of the population undergoing coronary revascularization: those with ESRD and those with heart failure.
 Epidemiology of Acute Kidney Injury
Epidemiology of Acute Kidney Injury
Definitions of Acute Kidney Injury
Several definitions have been used to identify AKI, resulting in wide variation in estimates of its incidence. Within the context of contrast-induced AKI, the interventional cardiology and radiology literature commonly define contrast-induced AKI as a rise in serum creatinine of at least 0.5 mg/dL or a 25% increase from baseline within 48 to 72 hours after the procedure.2,3 However, these definitions differ from those in the cardiothoracic and nephrology literature, which seeks to evaluate AKI in a variety of settings not limited to contrast exposure.4 The Society of Thoracic Surgeons defines postoperative renal insufficiency as a twofold or greater elevation of creatinine that must exceed 2.0 mg/dL, whereas renal failure is defined as AKI requiring dialysis.5 In contrast, definitions from the nephrology community include graded criteria for AKI by the Acute Kidney Injury Network group (AKIN criteria) and the Acute Dialysis Quality Initiative (RIFLE criteria, including stages of Risk, Injury, Failure, Loss, and End stage).6,7 In an attempt to standardize the definition of AKI across the scientific community, these systems grade AKI on the basis of urine output or change in creatinine from baseline, with the mildest stage of AKI defined as the rapid development (<48 hours) of renal dysfunction, including either a rise in serum creatinine (absolute rise ≥0.3 mg/dL [≥26.4 micromol/L] or relative rise ≥50% from baseline) or a reduction in urine output to less than 0.5 mL/kg per hour for >6 hours. The severity of AKI can be further staged based on the magnitude of increase in serum creatinine or reduction in urine output. Although some studies have sought to determine the relative advantages between these criteria, there remains no clear consensus.8,9 Greater recognition of this problem has led to several reports based on data from the Contrast-Induced Nephropathy Consensus Working Panel, an international multidisciplinary group that convened to address the challenges of contrast-induced AKI.10
Incidence of Acute Kidney Injury
The incidence of AKI depends on both the population studied and the definition used. Using a common definition of contrast-induced nephropathy (a rise in serum creatinine levels of 0.5 mg/dL or a 25% increase from baseline), the reported incidence ranges from 8% to 15% in the general population11 and up to 28% in those with acute coronary syndromes (ACSs).12
Prognosis of Acute Kidney Injury
In most cases, AKI after cardiac catheterization and PCI is completely reversible, with a typical clinical course consistent with acute tubular necrosis and nonoliguric AKI. Abnormalities in serum creatinine levels start within 24 to 48 hours after the procedure, peak at 5 days, and then completely recover within 2 to 4 weeks.13 The need for renal replacement therapy with hemodialysis or peritoneal dialysis is rare.14 Among those who do require renal replacement therapy (1%–4%), less than 50% require it permanently.10 The requirement for renal replacement therapy appears to be more likely in the setting of renal atheroembolic disease, which has a more progressive course than contrast-induced nephropathy and a lower likelihood of recovery.
Importantly, the development of AKI after cardiac catheterization and PCI has been associated with several clinical outcomes unrelated to renal disease, including longer hospital stays and greater inpatient costs.15 Recent reports also suggest that the development of contrast-induced nephropathy predicts short- and long-term mortality.16–19 What remains unclear from this literature, however, is whether the development of AKI after PCI is simply a marker of greater disease acuity or additional comorbidities like diabetes mellitus.
Pathophysiology of Contrast-Induced Aki
The most common reason for AKI after cardiac catheterization and PCI is related to the use of intravascular contrast agents. Despite their widespread use in imaging studies, however, the exact mechanisms responsible for the development of contrast-induced nephropathy remain unknown.1 Most studies suggest that both (1) direct toxic injury to the renal tubules and (2) ischemic injury to the renal medulla from vasomotor changes and decreased perfusion are responsible. The latter appears to be mediated partly by the development of reactive oxygen species like superoxide and has important implications for treatment with scavenging agents.20 Diabetes mellitus and heart failure may also exacerbate contrast-induced nephropathy, specifically by impeding vasodilatory responses in the renal vasculature (Table 5-1).21 However, these mechanisms are often insufficient in the absence of reduced renal function. The presence of CKD, or a reduction in functional renal mass, appears to be necessary for these mechanisms to cause AKI from ischemic injury. In addition, this risk appears to be multiplied in the presence of diabetes.10 A much less common but well-recognized cause of AKI after cardiac catheterization and PCI is renal atheroembolic disease. This disease process is part of the larger cholesterol embolization syndrome, which can result from the embolism of minor atheromatous debris from the aorta or other large vessels and its movement into small arteries in different vascular beds.22 The clinical spectrum of renal atheroembolic disease includes blue toe syndrome, livedo reticularis, visual deficits, and abdominal pain from mesenteric ischemia.22 Laboratory abnormalities include elevated eosinophil counts in the blood and eosinophiluria. AKI is believed to be caused by distal and partial occlusion of the small arteries, leading to ischemic atrophy as opposed to large areas of infarction.23 Treatment for renal atheroembolic disease is largely supportive. Finally, additional factors may exacerbate the development of AKI after cardiac catheterization and PCI. Many medications may directly contribute to renal toxicity or worsen microvascular changes in the renal medulla, thus extending areas of ischemic injury. Table 5-2 lists several of these agents as well as others that should be monitored closely owing to their potential interactions with contrast agents.24 For example, metformin can cause lactic acidosis in the setting of renal dysfunction; this has led the Food and Drug Administration to recommend withholding it on the day of exposure to contrast agents and for 48 to 72 hours after. Similarly, volume depletion and hemodynamic changes from heart failure or cardiogenic shock may aggravate contrast-induced nephropathy. In case reports, anticoagulants like warfarin and heparin, given their potential to prevent proper healing of atheromas in the aorta after instrumentation, have been implicated as causative agents in renal atheroembolic disease.23,25
TABLE 5-1 Risk Factors for the Development of Contrast-Induced Nephropathy
| Clinical factors |
| Presenting factors |
TABLE 5-2 Concomitant Drugs to Monitor with Exposure to Contrast Agents
| Drugs influencing renal hemodynamics |
| Drugs that cause tubular toxicity |
| Drugs with potentially enhanced toxicity after contrast-induced nephropathy |
Adapted from Erley C. Concomittant drugs with exposure to contrast media. Kidney Int. 2006;100:S20–S24.
 Risk Factors for Contrast-Induced AKI
Risk Factors for Contrast-Induced AKI
Several bedside tools have been created to predict a patient’s risk of developing contrast-induced nephropathy after cardiac catheterization and PCI.26,27 One model by Mehran and colleagues, developed in 8,357 patients undergoing PCI, uses eight readily available variables to calculate an overall risk score for predicting both the risk of contrast-induced nephropathy and nephropathy requiring dialysis (Fig. 5-1).27 Variables in this model are scored from 1 to 6 and then summed to generate risks of contrast-induced nephropathy ranging from 7.5% to 57.3% and risks of nephropathy requiring dialysis from 0.04% to 12.6%. The use of models such as these allows clinicians to appropriately discuss the potential benefits and risks of cardiac catheterization with high-risk patients prior to their procedures. They also may help target potential strategies to minimize the risk of developing contrast-induced AKI. One of the most powerful predictors of AKI following cardiac catheterization is the presence of preexisting CKD; in most cases with long-term complications, patients have preexisting evidence of advanced CKD. The risk of developing AKI following cardiac catheterization increases with increasing severity of CKD, such that patients undergoing PCI with a baseline serum creatinine >1.5 mg/dL or an estimated glomerular filtration rate (eGFR) of <60 mL/min per 1.73 m2 have an expected 30% incidence of contrast-induced nephropathy with an adjusted odds ratio of 2.05 (95% CI 1.59-2.66) of developing contrast-induced nephropathy.27 In addition to CKD, several other risk factors for developing renal dysfunction after cardiac catheterization and PCI have been identified (Table 5-1). Most importantly, these appear to be related to demographics like advanced age, comorbidities like diabetes mellitus, periprocedural factors like hemodynamic instability or heart failure, and evidence of volume depletion.28 Additional factors include the use of intra-aortic balloon pumps and nephrotoxic medications like nonsteroidal anti-inflammatory drugs (NSAIDs).
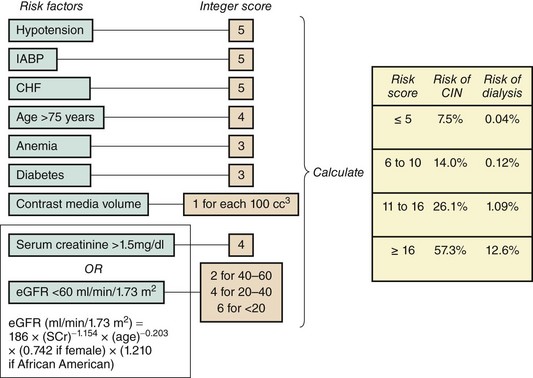
Figure 5-1 Risk score for determining risk of contrast-induced nephropathy and dialysis following PCI.
(Adapted from Mehran R et al, A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393.)
Chronic Kidney Disease
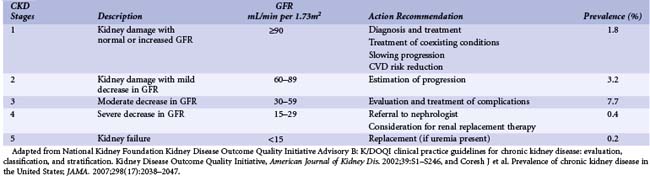
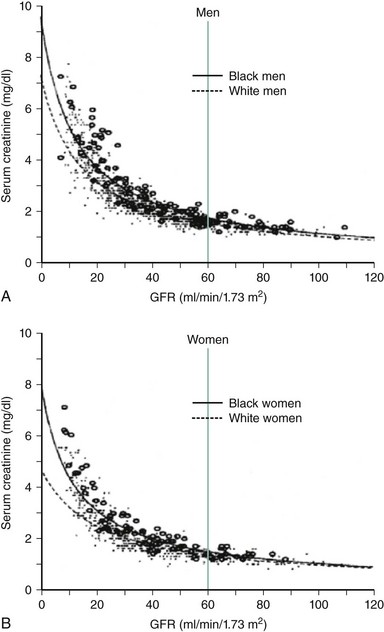
The population of patients with CKD worldwide is growing at a tremendous rate. Consequently, these high-risk patients are now encountered much more frequently in the cardiac catheterization laboratory. In one recent registry, for example, 25% of patients undergoing PCI had at least mild CKD.29 For the interventional cardiologist, identifying these patients is important for two reasons. First, this group represents those patients who are at highest risk for developing renal dysfunction following PCI and require specific preventive therapies prior to their procedures. Second, patients with CKD at baseline are also more likely to have worse cardiovascular outcomes after their procedures. This latter finding is due in part to the well-established relationship between CKD and cardiovascular disease. Until recently, defining patients with CKD was problematic owing to a multitude of nonstandardized definitions and inaccurate assessments of glomerular filtration rates (GFR). The National Kidney Foundation now specifically defines CKD as the presence of sustained abnormalities of renal function, manifest by either a reduced GFR or the presence of kidney damage.30 Kidney damage is defined by structural or functional abnormalities of the kidney in the presence or absence of decreased GFR manifest by either pathological abnormalities (assessed by renal biopsy) or markers of kidney damage including laboratory abnormalities (in the composition of blood or urine) and radiographic abnormalities (on imaging tests).30 Once GFR has been assessed, patients with CKD can be stratified into one of five stages (Table 5-3) in order of increasing impairment: stage 1 (GFR ≥90 mL/min per 1.73 m2), stage 2 (GFR 60-89 mL/min), stage 3 (GFR 30-59 mL/min), stage 4 (GFR 15-29 mL/min), and stage 5 (GFR <15 mL/min). Patients with GFRs of ≥60 mL/min are considered to have CKD if they meet additional criteria, demonstrating evidence of kidney damage based on pathological, laboratory, or imaging tests. Such markers of kidney damage include microalbuminuria, proteinuria, abnormalities of the urinary sediment, or abnormal radiological findings. In all cases, CKD requires that kidney disease has persisted for 3 months or longer. Importantly, a normal serum creatinine does not necessarily reflect normal kidney function, and standard reference ranges for normal often misclassify patients with early disease (Fig. 5-2).31 Such errors result from the fact that serum creatinine alone does not accurately reflect the level of GFR because of nonlinear relationships that vary according to age, gender, race, and lean body mass. Both direct and indirect measures of GFR are available. Direct measurements of GFR may be more accurate, but they are impractical in routine clinical practice. Indirect measurements of GFR are obtained by incorporating serum creatinine values into formulas such as the Cockcroft-Gault equation or the equation of the Modification of Diet in Renal Disease (MDRD) study.30 Although the MDRD study equation has generally been purported to have less bias, both formulas have limitations in accuracy, especially for patients with normal kidney function.32 In addition, these formulas do not perform well for many other individuals who were not well represented in the cohorts from which these equations were developed, including those who have very high or low muscle mass, weight, or age; are severely ill or hospitalized; ingest no or large amounts of meat; or are from minority racial and ethnic groups, such as Asians or Hispanics.32 Nonetheless in the ACS setting, more conservative estimation of kidney function using the Cockcroft-Gault equation is preferable for drug dosage adjustments so as to minimize overdosing, which may otherwise increase the risk of bleeding in high-risk groups including women and the elderly.33 In addition to developing AKI, patients with CKD are at particularly high risk for death and adverse cardiovascular events following interventional procedures.34–36 The risk of adverse outcomes is progressive, with an independent, graded association between reduced GFR and risk of hospitalizations, cardiovascular events, and death.34,36,37 Consequently, the National Kidney Foundation, American Heart Association, and the Seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure have classified the presence of CKD as a cardiovascular risk factor.30,35,38
Mechanisms by which CKD increases cardiovascular risk are unclear and under investigation. The progressive increase in cardiovascular risk associated with declining kidney function is largely explained by a larger burden of traditional risk factors.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree