Continuous venovenous hemofiltration (CVVH) is a renal replacement therapy that has been successfully used in patients with severe chronic renal failure to prevent contrast-induced acute kidney injury (CI-AKI). In this study, we present a consecutive experience using a new CVVH protocol that has also been applied to patients with acute coronary syndrome (ACS). CVVH was performed in consecutive patients with estimated glomerular filtration rate <30 ml/min/1.73 m 2 (mean ± SD, 21.1 ± 7.3 ml/min/1.73 m 2 ) undergoing diagnostic or interventional coronary procedures starting after the angiographic procedures. Iopamidol was used as a contrast agent. In the first 6 patients, iopamidol removal by the CVVH hemofilter and kidney was calculated by measuring iopamidol concentrations in the blood, urine, and ultrafiltrate collected during the 6-hour CVVH session. In the second phase, the protocol was applied to 47 additional patients meeting the inclusion criteria. Six-hour CVVH resulted in iopamidol removal comparable with that of 12-hour diuresis (43 ± 12% vs 42 ± 15% of administered, p = NS). CI-AKI occurred in 7.5% of patients in the whole population and no patients had acute pulmonary edema, need for dialysis, or any major bleeding. In conclusion, in a population including patients with ACS with severe chronic renal failure undergoing coronary angiographic procedures, 6-hour CVVH performed only after contrast medium exposure was able to remove an amount of contrast medium similar to that removed by the kidneys in 12 hours and resulted in a low rate of CI-AKI.
Contrast-induced acute kidney injury (CI-AKI) is associated with early and long-term mortality. For patients with severe chronic renal failure with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m 2 , further worsening of renal function may result in the need for long-term dialysis. Such patients experience high rates of atherothrombotic disease manifestation and may clinically present with acute coronary syndrome (ACS), frequently needing coronary angiography followed by percutaneous coronary intervention on an urgent basis. In this high-risk population, measures that were believed to prevent CI-AKI have produced only minimal effects. On the other hand, continuous venovenous hemofiltration (CVVH), a form of renal replacement therapy, has been successfully used in patients with severe renal failure according to protocols that include 6-hour utilization before administration of contrast medium, which makes this protocol unsuitable for patients with ACS who need an urgent intervention. In this study, we present a consecutive experience using a new CVVH protocol that has also been applied to patients with ACS.
Methods
Consecutive patients with eGFR <30 ml/min/1.73 m 2 measured by Modification of Diet in Renal Disease formula, undergoing angiographic cardiac procedures, were enrolled in the study. Patients with stable coronary disease undergoing elective procedures and patients with ACS (unstable angina, ST elevation myocardial infarction [STEMI], and non-STEMI) requiring urgent diagnostic procedures or interventions could be included in the study.
Criteria for exclusion were history of serious reactions to contrast medium, end-stage renal disease treated by long-term dialysis, kidney transplantation, recent major bleeding, contraindications to anticoagulation, and presentation with cardiogenic shock.
Interventional procedures were performed according to standard techniques at the discretion of each interventional cardiologist. The type of implanted stents (either bare metal or drug eluting) and number of stents were left to operators’ discretion. Low-osmolality contrast medium Iopamidol (Iopamiro 300; Bracco, Milan, Italy) was used during the procedures and the administered dose was recorded. Before the procedure, all patients were prescribed aspirin at a dose of 75 to 100 mg/day and clopidogrel at a loading dose of 300 or 600 mg.
In preparation of angiographic procedures, all stable patients were given intravenous saline solution at the rate of 1.2 to 1.6 ml/min, according to the clinical conditions and left ventricular function, for 12 hours before iopamidol exposure and lasting 24 hours after the end of CVVH. Moreover, these patients received oral N-acetylcysteine 1,200 mg twice daily, starting 24 hours before and lasting 48 hours after iopamidol administration. Furosemide was given intravenously concomitantly to hydration at a dose adjusted according to urine output. In patients with ACS needing urgent invasive treatment, intravenous saline infusion before the procedure could be <12 hours (mean time for infusion 140 ± 60 minutes) and oral N-acetylcysteine 1,200 mg could be administered only immediately before the diagnostic or interventional procedures.
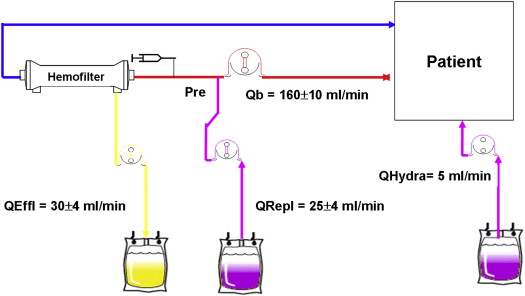
CVVH was performed through a double-lumen intravenous femoral catheter placed before the angiographic procedures, using Prisma or Prismaflex Gambro monitor (Gambro, Lund, Sweden). CVVH started immediately after the return to the coronary care unit after the angiographic procedures and lasted 6 hours. Blood flow was 160 ± 10 ml/min. Replacement fluid was infused by predilution mode at the mean rate of 25 ± 4 ml/min. The replacement fluid composition included Na + 142, K + 2, Cl − 113.5, HCO 3 − 32, Mg ++ 0.5, Ca ++ 1.75, and acetate 3 mmol/L. The hemofilter was a Gambro AN 69ST hollow fiber membrane with 0.6 m 2 surface. A loading heparin bolus of 5,000 IU was administered intravenously before CVVH initiation and followed by a continuous heparin infusion of 500 to 1,000 IU/hour through the arterial side of the circuit. During CVVH, all patients received 2,000 ml of normal saline infusion in a peripheral vein on the opposite side of the inserted double-lumen catheter for CVVH. In all cases, the ultrafiltration rate was matched to obtain a net fluid loss equal to the given saline infusion during CVVH treatment (2,000 ml; Figure 1 ).
In the first phase, in 6 patients meeting the inclusion criteria after elective percutaneous coronary intervention, the iopamidol concentration was measured in the ultrafiltrate collected during the entire CVVH session. The kidney iopamidol removal was evaluated by measuring its concentration in the whole urine volume obtained from the time of iopamidol administration to the end of CVVH and in the urine volume collected during the 6 hours after CVVH. The total amount of infused iopamidol (expressed in grams) was calculated by multiplying the infused volume (milliliter) with the iopamidol concentration (gram per milliliter). Likewise, the amount of iopamidol removed by either CVVH or the kidney was calculated by multiplying the ultrafiltrate and urine volumes with the respective iopamidol concentrations. Iopamidol concentrations were also measured in the plasma and ultrafiltrate samples collected at 15 and 360 minutes from the start of CVVH, to evaluate the iopamidol sieving coefficient, calculated as the ratio between the ultrafiltrate and the plasma concentrations, which indicates the hemofilter removal capacity for a target solute.
In the second phase, all consecutive patients meeting the inclusion criteria underwent CVVH according to the protocol. The primary end point of the study was the incidence of CI-AKI, defined either as an increase in creatinine concentration ≥0.5 mg/dl or ≥25% above the baseline value at 48 hours after the end of CVVH. Secondary end points were eGFR and creatinine values at hospital discharge and the need for dialysis due to CI-AKI. A written informed consent was obtained from all patients before undergoing CVVH. The Mehran score for the risk of CI-AKI was calculated for all patients included in the study. Major or minor bleeding were assessed using the Thrombolysis in Myocardial Infarction (TIMI) criteria. The protocol was approved by the local ethics committee.
Continuous variables are presented as mean ± SD or as median (interquartile range). Categorical data are presented as percentages and were compared using the chi-square or Fisher’s exact test, as appropriate. All tests were 2-tailed, and a p value of <0.05 was required for statistical significance. All calculations were computed with the aid of the SAS software package (version 9.13; SAS Institute Inc., Cary, North Carolina).
Results
Iopamidol concentrations in plasma and ultrafiltrate after 15 and 360 minutes of CVVH in 6 patients (mean age 78.1 ± 10 years, mean body weight 66.3 ± 3.5 kg, mean creatinine level 3.3 ± 0.6 mg/dl, and mean eGFR 22.5 ± 5.3 ml/min/1.73 m 2 ) are listed in Table 1 . The mean plasma iopamidol concentration decreased by 57% from 15 to 360 minutes after starting CVVH concomitantly with a 55% decrease of ultrafiltrate iopamidol concentration during the same period of time. The sieving coefficient was 85% ± 9%, indicating a large iopamidol transfer from blood to the hemofilter. As listed in Table 2 , 6-hour CVVH treatment resulted in iopamidol removal comparable to that of 12-hour diuresis (43 ± 12% vs 42 ± 15%; p = NS). The residual plasma iopamidol concentration at 12 hours was 12.6 mg/ml, equivalent to 15% of the administered dose ( Figure 2 ).
| Patient | Plasma Concentration (mg/ml) | Ultrafiltrate Concentration (mg/ml) | Average Sieving Coefficient | ||
|---|---|---|---|---|---|
| CVVH Time | 15 minutes | 360 minutes | 15 minutes | 360 minutes | |
| No. 1 | 11.5 | 3.15 | 8.94 | 3.29 | 91 |
| No. 2 | 5.01 | 2.64 | 3.85 | 2.32 | 82 |
| No. 3 | 5.1 | 2.45 | 3.7 | 2.05 | 78 |
| No. 4 | 4.74 | 1.99 | 3.46 | 1.58 | 76 |
| No. 5 | 11.5 | 5.13 | 11.27 | 4.49 | 98 |
| No. 6 | 4.66 | 2.37 | 3.73 | 1.97 | 82 |
| Median (Q1–Q3) | 5.05 (4.8–8.9) | 2.54 (2.3–3) | 3.8 (3.7–7.6) | 2.18 (1.9–3) | 82 (78–88) |
| Patient | eGFR Baseline (ml/min/1.73 m 2 ) | Infused Iopamidol (g) | 6-H CVVH Removal, g (%) | 6-H Kidney Removal, g (%) | 12-H Kidney Removal, g (%) | 12-H Diuresis (ml/h) | Residual Iopamidol (g) at 12 h, (%) |
|---|---|---|---|---|---|---|---|
| No. 1 | 24 | 147.7 | 66.3 (45) | 52.3 (37) | 74.7 (51) | 158.33 | 6.7 (5) |
| No. 2 | 23 | 73.8 | 28.7 (39) | 32.8 (44) | 42.8 (58) | 123.3 | 2.4 (3) |
| No. 3 | 20 | 80.6 | 22.5 (28) | 16.6 (20) | 24.6 (30) | 135 | 33.5 (42) |
| No. 4 | 26 | 58.5 | 24.4 (42) | 21.1 (36) | 32.0 (55) | 125 | 2.1 (4) |
| No. 5 | 28 | 104.6 | 68.2 (65) | 27.8 (27) | 35.7 (34) | 129.1 | 0.8 (1) |
| No. 6 | 13 | 73.8 | 27.7 (38) | 11.3 (15) | 15.9 (22) | 104.1 | 30.2 (41) |
| Median (Q1–Q3) | 23 (21–25) | 125 (120–160) | 28 (25–57) | 24.5 (18–31) | 34 (26–41) | 130 (127–132) | 4.5 (2.1–24) |
Table 3 lists the clinical and laboratory characteristics of the patients. Mean eGFR of the whole population was 21 ± 7 ml/min/1.73 m 2 . Mean Mehran score was 11.6 ± 3. In the subgroup of 17 patients with ACS who underwent urgent procedures for non-STEMI (15 patients) or STEMI (2 patients), the mean eGFR was 23 ± 8 ml/min/1.73 m 2 , and a Mehran score >11 was present in 14 patients (82%). In the 25 patients who had an eGFR <20 ml/min/1.73 m 2 (47%), 6 were patients with ACS undergoing urgent procedures.
| Variable | No. of Patients, n = 53 (%) |
|---|---|
| Sex, female | 18 (34) |
| Age, yrs (mean ± SD) | 73.8 ± 8.6 |
| Body weight, kg (mean ± SD) | 68.9 ± 9.7 |
| Diabetes mellitus | 31 (58) |
| Hypertension | 44 (83) |
| Total cholesterol >200 mg/dl | 20 (38) |
| Current smoker | 8 (15) |
| Peripheral vascular disease | 20 (38) |
| Previous myocardial infarction | 29 (54) |
| Previous percutaneous or surgical coronary revascularization | 26 (49) |
| Mehran score >11 | 35 (66) |
| Total diagnostic coronary angiographic procedures | 53 (100) |
| Total percutaneous coronary interventions | 43 (81) |
| Urgent procedures for patients with ACS | 17 (32) |
| Killip class >II | 8 (51) |
| Baseline creatinine level, mg/dl (mean ± SD) | 3.1 ± 1.03 |
| Baseline eGFR, ml/min (mean ± SD) | 21.1 ± 7.3 |
| eGFR <20 ml/min/1.73 m 2 | 25 (47) |
| Left ventricular ejection fraction (mean ± SD) | 46.9 ± 9.5 |
| Left ventricular ejection fraction <40% | 11 (21) |
| Long-term diuretic use | 40 (75) |
| Iopamidol dose infused, ml (mean ± SD) | 139.1 ± 91 |
| Iopamidol infused in urgent procedures, ml (mean ± SD) | 114 ± 54 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


