62 Connective Tissue Diseases and the Heart
Syndromes
Rheumatoid Arthritis
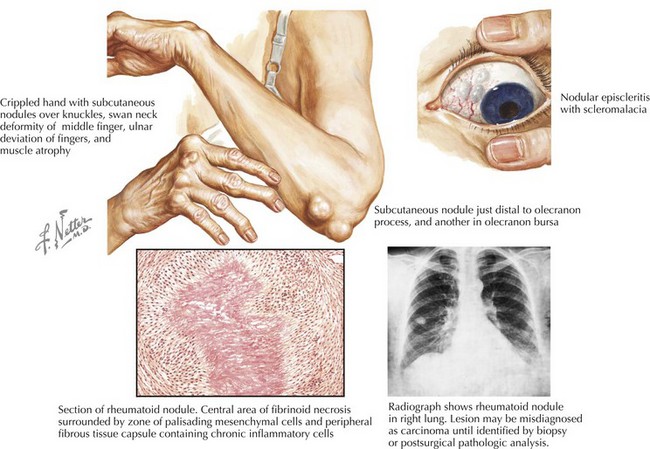
RA, characterized by a symmetric, additive, destructive synovitis, occurs in 1% of most populations. The most frequent cardiac manifestations in patients with RA are pericarditis and valvular heart disease (Fig. 62-1). These features are more common in patients with nodular seropositive RA than in RA patients without extra-articular pathology. With routine screening echocardiograms, pericardial thickening with or without a pericardial effusion may be seen in up to 60% of patients, though clinically evident in less than 5% (Tables 62-1 and 62-2). Pericardial fluid due to RA involvement is exudative, serosanguineous, or hemorrhagic with high acidity. Adhesions and loculations are common, often making pericardiocentesis ineffective. A significant proportion of patients with clinical pericarditis have constriction or tamponade with a grave prognosis. These patients, under some circumstances, may benefit from surgical pericardiectomy.
Table 62-1 Clinical Cardiac Manifestations in Rheumatologic Disorders
| Disorder | Common | Less Common/Rare |
|---|---|---|
| Rheumatoid arthritis | ||
| Systemic lupus erythematosus | ||
| Ankylosing spondylitis | ||
| Inflammatory myopathy | ||
| Scleroderma | ||
| Antiphospholipid syndrome |
Table 62-2 Prevalence of Cardiac Involvement in Rheumatologic Disorders
| Disorder | Noninvasive Tests | Autopsies |
|---|---|---|
| Rheumatoid arthritis | ||
| Systemic lupus erythematosus | ||
| Ankylosing spondylitis | Aortic root thickening and dilation 20% to 60% | |
| Inflammatory myopathy | Myocarditis 30% | |
| Scleroderma |
ECG, electrocardiogram; Echo, echocardiogram; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.