Congenitally Corrected Transposition of the Great Arteries (Atrioventricular and Ventriculoarterial Discordance)
Joseph Atallah
Jennifer M. Rutledge
John D. Dyck
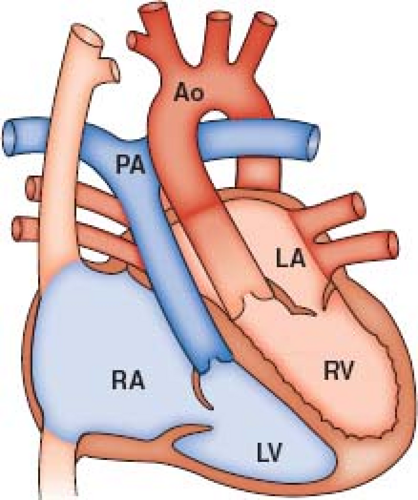
The language of corrected transposition can be difficult. Isolated ventricular inversion, double discordance, physiologically corrected transposition, and l-transposition are all terms that have been applied to congenitally corrected transposition of the great arteries (ccTGA). For the patient with ccTGA, situs solitus, and normal atrial arrangement, the systemic venous return joins the morphologic right atrium. This atrium is connected by a mitral valve (MV) with the morphologic left ventricle (LV), which in turn supports a discordantly connected, transposed pulmonary artery (PA) (Fig. 48.1). The left atrium, receiving the pulmonary veins, connects through a tricuspid valve (TV) with the morphologic right ventricle (RV), which in turn supports a transposed aorta (Fig. 48.1). These atrioventricular (AV) and ventriculoarterial (VA) connections do occur in the setting of situs inversus. Patients with other abnormalities of situs and other AV connections are properly excluded from discussion here (1,2).
In ccTGA, the effects of ventricular inversion on blood flow are physiologically “corrected” by the associated transposition of the great arteries (TGA). That is, the path of systemic venous blood returning to the heart leads to the PA and the path of pulmonary venous return leads to the aorta. However, even in the patient with no associated abnormalities, it is increasingly apparent that natural history and hemodynamics will be far from normal (3).
Prevalence, Etiology, and Morphogenesis
Congenitally corrected transposition is an uncommon lesion. Data from several sources would suggest a prevalence of 0.03 per 1,000 live births accounting for approximately 0.4% of congenital heart malformations and 4% of conotruncal defects (4,5,6). The majority of these patients will have situs solitus, and about 5% will have situs inversus (7).
Population-based studies continue to support the possible importance of environmental factors in the etiology of this condition (8). Still, the familial occurrence and molecular biology investigations suggest the importance of a genetic influence (9,10). It would seem wise therefore to continue to counsel a multifactorial etiology with a congenital heart disease recurrence risk in first-degree relatives of approximately 2% to 5% (4,9,11). Conotruncal defects, including d-TGA, and other forms of congenital heart defects have been identified in affected first-degree relatives (4,9).
Morphogenetically, the primitive cardiac tube, anchored at one end by the sinus venosus and at the other end by the truncus arteriosus, loops to the left (l-looped) and not to the right (d-looped) as in the normal heart (12). This abnormal cardiac looping brings the morphologic LV to the right and the morphologic RV to the left. The origins of abnormal cardiac looping continue to be an area of active investigation (13,14). Most frequently, such abnormal looping of the ventricles is associated with a transposed VA connection.
Morphology
Despite the relative rarity of ccTGA, important studies and descriptions of the basic morphology have been published for some years (15,16,17,18). Advances in the surgical management of ccTGA have occasioned reassessments of the pertinent anatomy (19,20).
As noted, some 5% of patients with ccTGA will have situs inversus (7). Furthermore, approximately 25% of patients will demonstrate either dextrocardia or mesocardia (17). The ventricular topology (relative orientation of the ventricles) in ccTGA places the RV to the left of the morphologic LV. As a result of the abnormal looping, the ventricles conform to a left hand pattern (21). That is, if one places the palm of the hand against the right ventricular surface of the interventricular septum, the thumb in the inlet and the fingers in the outlet, it is necessary to use the left hand in ccTGA in contradistinction to the right hand in the normally d-looped ventricles. Further twisting can result in a more superior-to-inferior relationship of the RV to the LV (22). The interventricular septum tends to a more sagittal or horizontal position resulting in one of the central features of AV discordance, that of malalignment of the atrial
and ventricular septum. The atrial septum and ventricular septum meet at the crux of the heart. However, as the atrial septum continues anterior and to the right, it will deviate to a variable degree from the ventricular septum creating a variable gap that in extreme cases will go back as far as the crux (16). This concept of malalignment of the atrial and ventricular septum has implications for the size and extent of the ventricular septal defect (VSD), the ventricular outflows, and the conduction system (23).
and ventricular septum. The atrial septum and ventricular septum meet at the crux of the heart. However, as the atrial septum continues anterior and to the right, it will deviate to a variable degree from the ventricular septum creating a variable gap that in extreme cases will go back as far as the crux (16). This concept of malalignment of the atrial and ventricular septum has implications for the size and extent of the ventricular septal defect (VSD), the ventricular outflows, and the conduction system (23).
The AV valve on the right side has the features of an MV with two papillary muscles and no insertion onto the interventricular septum. Penny et al. (24) have pointed out that 10% of MVs in this setting will demonstrate significant echocardiographic abnormalities. On the left, the AV valve has features of a TV. This valve frequently is abnormal with anterior positioning bringing the septal leaflet into the “gap” created by the septal malalignment at the membranous septum. This leaflet may thus form one wall of the left ventricular outflow tract (LVOT) (16).
Most frequently, AV discordance is associated with TGA and a leftward anterior position of the aortic valve relative to the pulmonary valve, though this is not absolute (21). Freedom et al. (25) have shown that leftward anterior positioning of the aorta also may occur in the setting of complete transposition, double-outlet RV, anatomically corrected malposition, crossed AV connections, superoinferior ventricles, and univentricular connections (26). The LVOT is deeply wedged between the left and right AV valves and is therefore more readily subject to obstruction. The pulmonary valve is most often in fibrous continuity with the MV.
The leftward anterior aorta is supported by a muscular infundibulum and is not in fibrous continuity with either AV valve. Obstructive lesions of the right ventricular outflow tract and aorta perhaps have been underemphasized. Several reports suggest the higher frequency of this problem in association with severe left AV valve regurgitation. Systemic outflow obstruction may take the form of functional and/or true aortic valve atresia as well as obstructive anomalies of the aortic arch (27,28,29).
Associated Lesions
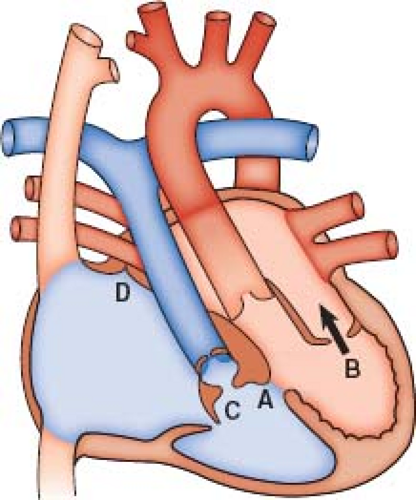
Patients with ccTGA and no associated abnormalities are in fact the exception, with associated anomalies occurring in well over 90% (3). The most frequent abnormalities include VSD, LVOT obstruction, and anomalies of the left-sided TV (Fig. 48.2).
Ventricular Septal Defect
VSDs occur in approximately 80% of hearts with ccTGA (18). The defects are most often perimembranous and a consequence of the atrial and ventricular septal malalignment (16). This places them in a subpulmonary position and in approximation to the septal leaflet of the left-sided TV. The defects are often large with anterior extension and therefore suitable for intraventricular tunneling. Other defects such as the subaortic or muscular defect do occur but are unusual.
Pulmonary Outflow Obstruction
Obstruction to the outflow tract of the morphologic LV is identified in 30% to 50% of patients with ccTGA and atrial situs solitus. Such pulmonary outflow tract obstruction rarely occurs in isolation at the valve or infundibular level, but usually is associated with a large VSD. In about one-third of patients with a VSD and pulmonary outflow tract obstruction, abnormalities of the morphologic TV are observed as well. The LVOT obstruction may be muscular, reflecting wedging of the subpulmonary outflow tract between the infundibular septum and the ventricular free wall, with contributions from the right-sided ventriculoinfundibular fold. Fibrous tissue derived from the membranous septum may participate in LVOT obstruction. Tissue tags derived from the tricuspid or mitral valve or stenosis of the pulmonary valve itself also may obstruct flow into the pulmonary trunk. Such tissue tags are likely the most common obstructive lesion (30).
Lesions of the Morphologic Tricuspid Valve
Abnormalities of the morphologic TV are intrinsic to hearts exhibiting ccTGA. Although at autopsy about 90% of hearts exhibit some abnormality of the morphologic TV, a clinically apparent functional disturbance during life is considerably less often manifested. The most common and important underlying pathology is dysplasia of the valve, with or without displacement of the septal or posterior leaflets of the TV. Anderson et al. (31) described the features associated with Ebstein-like anomaly of the left-sided TV and suggested, along with others, that these valves in general may represent a poor substrate for repair. Both the morphologic TV and on occasion the MV can be seen to straddle the ventricular septum. It is of course very important to recognize this anomaly preoperatively (32).
Coronary Artery Anatomy
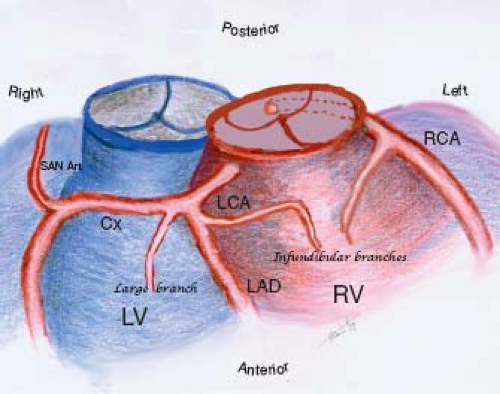
Changing approaches to the surgical management of ccTGA, including the so-called double-switch procedure, have refocused attention on the coronary artery anatomy. In general, the coronary arteries originate from the posterior-facing sinuses of the aortic valve. In patients with atrial situs solitus and ccTGA, the coronary arteries show a mirror-image distribution. The right-sided coronary artery has the epicardial distribution of a morphologic left coronary artery. The main right-sided coronary artery bifurcates into circumflex and anterior descending branches, whereas the left-sided coronary artery runs in the left AV groove and gives rise to infundibular and marginal branches (Fig. 48.3).
Several studies have demonstrated a variable pattern of coronary artery anomalies although the prevalent pattern is that of “coronary artery–ventricular concordance” (33). In a 14-specimen study (34), investigators observed the persistent origin of the sinus node artery from the circumflex artery. In that same report, a correlation between commissural malalignment and eccentric coronary ostia
was observed. Ismat et al. (35) analyzed 20 specimens and found 8 with eccentric ostia. Rare cases of an isolated origin of the sinus node artery from a coronary sinus have also been reported (36). The largest pathologic study (46 specimens) by Uemara et al. (36) reported a 76% incidence of a relatively “normal” pattern with the right and left coronaries originating from the left and right facing sinuses, respectively. Anomalies were found in 11 specimens and a single coronary artery was the most common in four (two originating from the right and two from the left facing sinuses). A main coronary branch coursing anterior to the pulmonary trunk was found in 96% of the specimens, and a large infundibular branch supplying the right ventricular outflow tract was found in 61% of the specimens. These findings are of most importance when considering a ventricular to PA conduit. The posterior descending artery was supplied by the morphologic right coronary artery in 59% of specimens. Chiu et al. (37) described a segmental approach to the coronary anatomy. From their study of 62 patients, they concluded that the proximal coronary pattern at the aortic sinus depends on the aortopulmonary rotation, and the peripheral coronary pattern depends on the atrial situs and apical position (apicocaval ipsilaterality), as well as ventricular looping (37). A good understanding of the type and degree of variability of the coronary anatomy in patients with ccTGA is essential in surgical planning.
was observed. Ismat et al. (35) analyzed 20 specimens and found 8 with eccentric ostia. Rare cases of an isolated origin of the sinus node artery from a coronary sinus have also been reported (36). The largest pathologic study (46 specimens) by Uemara et al. (36) reported a 76% incidence of a relatively “normal” pattern with the right and left coronaries originating from the left and right facing sinuses, respectively. Anomalies were found in 11 specimens and a single coronary artery was the most common in four (two originating from the right and two from the left facing sinuses). A main coronary branch coursing anterior to the pulmonary trunk was found in 96% of the specimens, and a large infundibular branch supplying the right ventricular outflow tract was found in 61% of the specimens. These findings are of most importance when considering a ventricular to PA conduit. The posterior descending artery was supplied by the morphologic right coronary artery in 59% of specimens. Chiu et al. (37) described a segmental approach to the coronary anatomy. From their study of 62 patients, they concluded that the proximal coronary pattern at the aortic sinus depends on the aortopulmonary rotation, and the peripheral coronary pattern depends on the atrial situs and apical position (apicocaval ipsilaterality), as well as ventricular looping (37). A good understanding of the type and degree of variability of the coronary anatomy in patients with ccTGA is essential in surgical planning.
Specialized Conduction Tissues
The conduction system in patients with ccTGA is abnormal and potentially unstable. Several investigators have helped elucidate the presence of normal and abnormal conduction tissues (17,38,39,40,41). The sinoatrial (SA) node lies in its normal position in relation to the atrial situs. The AV conduction tissue, on the other hand, is abnormal. The classic description is that of two AV nodes: (1) a normal posterior AV node located at the apex of the triangle of Koch but with no AV bundle, and (2) an abnormal right anterior AV node giving rise to the penetrating AV bundle. The latter is located anterosuperiorly in the area lateral to the pulmonary–mitral valve continuity, inferior and medial to the opening of the right atrial appendage. Its AV bundle has a superficial course along the anterior aspect of the subpulmonary outflow tract and LV wall. The bundle then courses onto the anatomic right side of the upper interventricular septum from which it descends and branches. The bundle branches remain associated with the morphologic ventricles, with the left bundle on the right and the right bundle on the left side of the septum. If a perimembranous VSD is present, the anterior AV bundle courses along its anterosuperior margin. Interestingly, in the more rare form of ccTGA with situs inversus, small case series have identified only a normally positioned posterior penetrating bundle (42,43,44).
Although still unclear, the etiology of the conduction system abnormalities in ccTGA may be related to abnormal ventricular looping and septal malalignment. It is hypothesized that the development of an AV bundle from the normal posterior AV node to the summit of the interventricular septum is anatomically hindered by the atrial and ventricular septal malalignment (38,39). A recent study described a positive correlation between the size of the pulmonary trunk and the degree of septal malalignment, suggesting an association with the conduction system location in ccTGA (23). In patients with both conduction systems straddling the anterosuperior and inferoposterior margins of a VSD, there can exist a sling-like bundle located over the anterior margin of the VSD and connecting both AV bundles, as described by Monckeberg (41). This electro-anatomical setup can mediate an AV re-entrant twin AV node tachycardia.
Clinical Features and Physical Examination
Modes of presentation of patients with ccTGA are highly variable largely based on the presence of associated lesions. Increasingly, the diagnosis is made antenatally (45). Patients with isolated ccTGA will be asymptomatic in childhood and may be referred for evaluation of a murmur, a loud second heart sound, or bradycardia reflecting high-degree AV block. Others may not present until adulthood when right ventricular dysfunction, heart block, or other arrhythmias become apparent (3). On the other hand, patients with associated lesions will have a variable presentation. Infants may present with AV block, tachyarrhythmia, cyanosis and/or congestive heart failure. Congestive heart failure may reflect a cardiac arrhythmia, but more likely indicates a large VSD, significant regurgitation of a dysplastic or displaced left-sided morphologic TV, obstructive anomalies of the aortic arch, or a combination of these anomalies.
As with the variable clinical presentation of patients with ccTGA, the physical examination reflects the presence and severity of associated lesions (46). Characteristically patients have an accentuated, often palpable, single second heart sound reflecting the anteriorly positioned aortic valve. Patients are acyanotic unless severe pulmonary stenosis is present along with a VSD. Other physical findings reflect associated lesions (e.g., VSD, pulmonary stenosis, or systemic AV valve insufficiency). The presence of findings suggestive of “mitral regurgitation” in a neonate should prompt consideration of ccTGA and an abnormal systemic AV valve.
Electrocardiographic Features
In the patient with normal atrial situs and discordant AV and VA connections, the direction of the frontal P-wave axis is normal and therefore positive in leads I, II, III, and aVF, but negative in aVR.
The electrical activation of the ventricles in the normal heart begins in the interventricular septum
from left to right and in a slightly anterior direction. This initial activation is responsible for the normal Q-wave pattern in precordial leads: qR in V6 and reciprocal RS in V1. The absence of Q waves in the left precordial leads is seldom observed in normal children, but 25% of normal neonates may not demonstrate a Q wave in V6. In ccTGA, the interventricular septum has a more or less sagittal disposition and is oriented from left posterior to right anterior. With ventricular inversion, both its surfaces and ventricular bundle branches are inverted, thus the sequence of initial activation is oriented from right to left and usually in a more superior and anterior direction. This results in a reversal of the normal Q-wave pattern in the precordial leads: Q waves are present in the right precordial leads but are absent in the left precordial leads. This pattern of reversal is appreciated less commonly when the heart is right sided or when there are confounding associated lesions producing pressure or volume overload (15).
from left to right and in a slightly anterior direction. This initial activation is responsible for the normal Q-wave pattern in precordial leads: qR in V6 and reciprocal RS in V1. The absence of Q waves in the left precordial leads is seldom observed in normal children, but 25% of normal neonates may not demonstrate a Q wave in V6. In ccTGA, the interventricular septum has a more or less sagittal disposition and is oriented from left posterior to right anterior. With ventricular inversion, both its surfaces and ventricular bundle branches are inverted, thus the sequence of initial activation is oriented from right to left and usually in a more superior and anterior direction. This results in a reversal of the normal Q-wave pattern in the precordial leads: Q waves are present in the right precordial leads but are absent in the left precordial leads. This pattern of reversal is appreciated less commonly when the heart is right sided or when there are confounding associated lesions producing pressure or volume overload (15).
If one were to catalog the electrocardiographic change identified in patients with ccTGA, they would include reversal of Q-wave disposition in the precordial leads with QS complexes in the right precordial leads, large Q waves in leads III and aVF, and left axis deviation.
With the precarious nature of the conduction system, as previously noted, the occurrence of congenital and postsurgical complete AV block is significant. Complete AV block is present in about 4% of patients at birth and the overall lifetime incidence is 20% to 30% (47,48). The most important feature is the increasing prevalence of complete heart block in the ccTGA population during follow-up, with an estimated rate of 2% per year after diagnosis (47,48). Despite the development of surgical techniques to reduce the incidence of complete heart block at surgery, this problem continues to be significant and progressive (49). It is imperative for this reason alone that all patients with ccTGA have long-term follow-up.
Radiographic Features
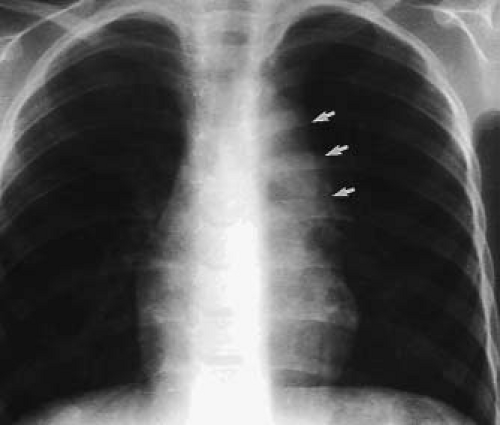
Although the chest radiograph is thought to provide useful information in the diagnosis and evaluation of the patient with ccTGA, these observations are probably more germane to the older infant or child than to the neonate with this disorder. The chest radiograph of the patient with levocardia, atrial situs solitus, and ccTGA may reflect the abnormal VA junction. The most common spatial relationship of the great arteries in ccTGA is a side-by-side or oblique one, with the aorta to the left. This is manifested in the plain chest radiograph in the frontal projection as a deformity of the left upper mediastinal border characterized by a convex prominence at its middle and upper portions with a mild convexity in the anticipated position of the pulmonary trunk (Fig. 48.4). This shadow represents the levopositioned ascending aorta, which originates from the left-sided morphologic RV. A levopositioned aorta is not diagnostic of discordant AV and VA connections. Other radiographic findings reflect the presence of associated lesions. A large left-to-right shunt from a VSD will result in cardiomegaly and increased pulmonary vascular markings. Similarly, cardiomegaly will be seen in the presence of significant insufficiency of the systemic AV valve.
Echocardiography
The examination of the patient with complex AV and VA connections should begin with the definition of situs, which can be achieved adequately by cross-sectional ultrasound examination of the great vessels in the abdomen. In the patient with situs inversus, the aorta lies on the right of the spine, with the inferior vena cava (IVC) on the left and the morphologic right atrium on the left. This is important since 5% of cases of ccTGA will occur in the setting of situs inversus. Patients with situs ambiguous will demonstrate either interruption of the IVC, or the aorta and IVC on the same side of the spine. By definition, these patients cannot demonstrate true ccTGA, but nevertheless, this may be important in the differential diagnosis. In some patients, despite an ambiguous atrial situs, the atria may be relatively well lateralized.
By moving up from the cross-sectional (horizontal) view of the abdominal vessels to the subcostal view of the heart, cardiac position can be determined accurately. This is crucial as 25% of patients with ccTGA will demonstrate either dextrocardia or mesocardia (Video 48.1). In addition, this approach should make clear other abnormalities of the spatial relationships of the chambers, such as crisscross AV connections and superoinferior ventricles.
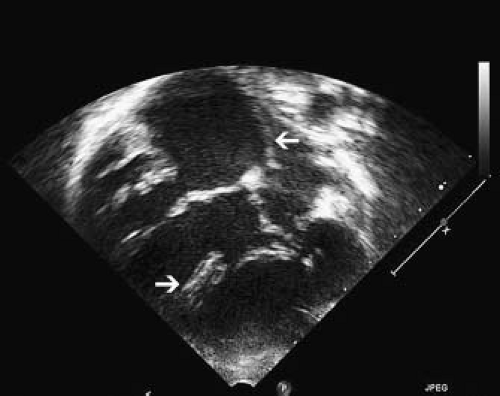
The subcostal views are important in the identification of a case of ccTGA because they allow imaging of all chambers and vessels. From this position, the first clue to the presence of AV discordance may be the significant malalignment between the atrial and ventricular septa that occurs in this condition (Fig. 48.5).
In all cases, features that define the morphologic RV versus LV should be evaluated. These features include the relative apical displacement of the AV valve in the RV (exaggerated in the presence of an Ebstein-like malformation and absent in the presence of an AV septal defect or a large perimembranous inlet VSD), a trileaflet (tricuspid) versus a bileaflet (mitral) AV valve, septal attachments
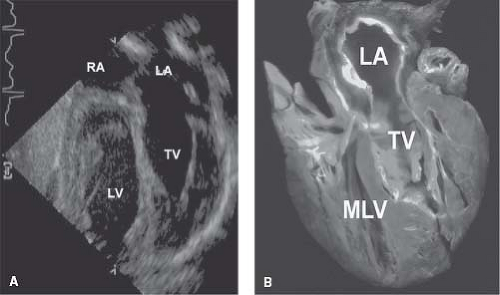
of the TV, the presence of a moderator band in the RV, an irregular mural endocardial surface of the RV relative to the smooth endocardial surface of the LV, and a round or triangular shape to the right ventricular cavity versus an elongated or ellipsoidal shape to the left ventricular cavity. The subcostal four-chamber view is important in evaluating these features (Fig. 48.6; Video 48.2).
of the TV, the presence of a moderator band in the RV, an irregular mural endocardial surface of the RV relative to the smooth endocardial surface of the LV, and a round or triangular shape to the right ventricular cavity versus an elongated or ellipsoidal shape to the left ventricular cavity. The subcostal four-chamber view is important in evaluating these features (Fig. 48.6; Video 48.2).
The long-axis views from the chest tend to be more vertically oriented than in the normal heart, and the two great arteries are seen to arise in parallel, confirming the presence of TGA. As the ventricular septum is often horizontally oriented in ccTGA, the long-axis views can be confusing, particularly in the presence of a large perimembranous inlet VSD. Nevertheless, the long-axis views are particularly important in establishing the mechanisms of possible outflow tract obstruction to either the aorta or PA (Fig. 48.7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree