Congenital Pulmonary Vascular Disease
Allen P. Burke, M.D.
Jennifer M. Boland, M.D.
Persistent Pulmonary Hypertension of the Newborn
Etiology and Clinical Findings
Persistent pulmonary hypertension of the newborn (PPHN) is characterized by markedly increased pulmonary vascular resistance without clear etiology. It is likely a heterogeneous group of disorders with various causes. Its estimated incidence is 1.9 per 1,000 live births.1
In utero, pulmonary vascular resistance is fairly high and falls rapidly with expansion of the lungs at birth, an 8- to 10-fold increase in pulmonary blood flow, and closure of the ductus arteriosus and fossa ovalis. The failure of arteriolar relaxation and hypoxemia constitutes PPHN. It is associated with impaired function of endothelial nitric oxide synthase and an increase in oxidative stress.2
Risk factors for PPHN include Black and Asian race, male gender, and maternal obesity, asthma, and diabetes.3
Clinically, infants are cyanotic and hypoxemic with markedly elevated pulmonary arterial pressures. There is poor cardiac output, which may lead to cardiogenic shock. By definition, there is a lack of cardiac shunting due to congenital defects, and persistent elevations of pulmonary vascular resistance cause right-to-left shunting across the ductus arteriosus or foramen ovale. Pulmonary hypertension caused by diseases of prematurity is considered a separate entity; therefore, PPHN is general not diagnosed in infants born <34 weeks gestation.
Conditions that predispose to PPHN include intrapartum asphyxia, infection, pulmonary hypoplasia, and congenital heart disease (excluding intracardiac shunts). There is an association with material use of nonsteroidal anti-inflammatory drugs, which is also implicated in premature ductal closure.4,5
Recently, PPHN has been grouped into three etiologic categories6 (Table 13.1). Although meconium aspiration syndrome is frequently mentioned in conjunction with PPHN, the frequency of PPHN in series of patients with meconium aspiration is low. Deaths are not related to PPHN, but rather intrauterine asphyxia with neurologic deficits, or associated congenital anomalies.7
Radiologic Findings
Chest x-ray is helpful in excluding associated conditions, including consolidation typical of meconium aspiration syndrome, congenital diaphragmatic hernia, and congenital pulmonary airway malformations (CPAMs). Pulmonary arterial hypertension is manifest by decreased vascular markings. Typically, the cardiac silhouette is normal.
Tissue Sampling
The diagnosis of PPHN is generally made clinically, based on pulmonary hypertension in a term infant, without structural heart disease. The diagnosis is confirmed at autopsy, by demonstration of extension of smooth muscle into distal arterioles.8
TABLE 13.1 Persistent Pulmonary Hypertension of the Newborn, Classification by Etiology | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Microscopic Findings
The hallmark of PPHN, especially the idiopathic type, is diffuse extension of medial smooth muscle to the precapillary pulmonary arteries9,10 (Fig. 13.1). Fully muscularized vessels extend as far as the intra-acinar arteries, which are normally nonmuscular at birth. In addition, there is medial hypertrophy of more proximal muscular arteries and arterioles. Morphometric analysis may be helpful in demonstrating medial hypertrophy.11 There are often superimposed changes related to infectious complications, including pneumonia and alveolar injury (diffuse alveolar damage). Significant meconium aspiration should lead to a diagnosis of meconium aspiration syndrome.
It is not entirely clear if the pulmonary vasculature in cases of PPHN associated with vasospasm or pulmonary hypoplasia shows similar features to the idiopathic form, including muscularization and medial hypertrophy. In a baboon model, neither meconium aspiration nor intrauterine asphyxia resulted in physiologic or histologic changes of PPHN.12
Immunohistochemical Stains
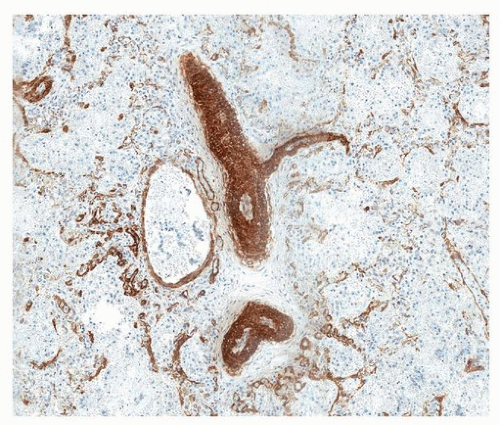
Immunohistochemical staining for smooth muscle actin highlights extension of muscular vessels into the alveolar septa (Fig. 13.2).
 FIGURE 13.1 ▲ Pulmonary hypertension of the newborn. Vessels within the capillary walls (alveolar septa) are focally thickened and muscularized. |
Differential Diagnosis
At autopsy, the main concern is to exclude associated conditions, such as congenital heart defects, pulmonary malformations, and alveolar capillary dysplasia (see below). From a clinical standpoint, the differential diagnosis includes congenital heart disease with left to right shunts, bronchopulmonary dysplasia and respiratory distress syndrome (seen in earlier gestational age), sepsis, and surfactant deficiency. Therefore, it is important for the autopsy pathologist to document an absence of evidence for these alternative conditions, in order to support a diagnosis of PPHN.
Prognosis and Treatment
Alveolar Capillary Dysplasia
Background and Clinical Findings
Alveolar capillary dysplasia is a rare lethal condition that was originally described in 1981 as a congenital absence of normal capillary ingrowth in the alveolar septa.13 Forty cases had been reported by 2000.14 Since then, there have been more than a dozen additional cases, some with emphasis on misaligned pulmonary veins as a component of the disease.15 The term “alveolar capillary dysplasia with misalignment of pulmonary veins” has been used to reinforce both components of the malformation.
Related conditions include severe developmental abnormalities such as acinar dysplasia and congenital alveolar dysplasia, both of which lead to severe and generally lethal pulmonary hypoplasia.16
A family history of similar disease has been reported in a little over 10% of cases, with several reports in siblings.14 There is no gender predominance, although one review found a 2:1 female bias.17 Most infants are full term, with presentations similar to PPHN, including respiratory distress and cyanosis. One-half of patients present within 24 hours, although symptoms may occur as late as 6 weeks. There is a high rate of extrapulmonary anomalies, most commonly involving the gastrointestinal tract (40% of patients) or genitourinary tract (one-third).17
Radiologic Findings
Chest radiograph may show normal findings, pneumothoraces, reticular markings, and granular, patchy, or diffuse opacities. Decreased pulmonary vascular markings are rarely seen. Echocardiography demonstrates a right-to-left shunt.17
Tissue Sampling
Microscopic Findings
The two histopathologic hallmarks of this disease are decreased capillaries in the alveolar septa and misplaced veins in the bronchovascular bundles (Fig. 13.3). Rare capillaries that are present in the alveolar septa are often centrally located, without the proper apposition to the type 1 pneumocytes required to facilitate gas exchange. The histologic appearance is that of alveolar septa with sparse capillaries (Fig. 13.4). Secondary changes of alveolar capillary dysplasia may include muscularization of distal arterioles and medial hypertrophy of small arteries. Bronchovascular bundles should normally only contain an airway, artery, and lymphatics; veins are normally localized to the interlobular septa, so presence of veins in the bronchovascular bundles is distinctly abnormal. The misplaced veins may be patchy, leading to a diagnosis of PPHN on biopsy and alveolar capillary dysplasia at autopsy. The misplaced veins are usually present between the arteries and airways.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree