Chapter 11 Congenital Pulmonary Stenosis
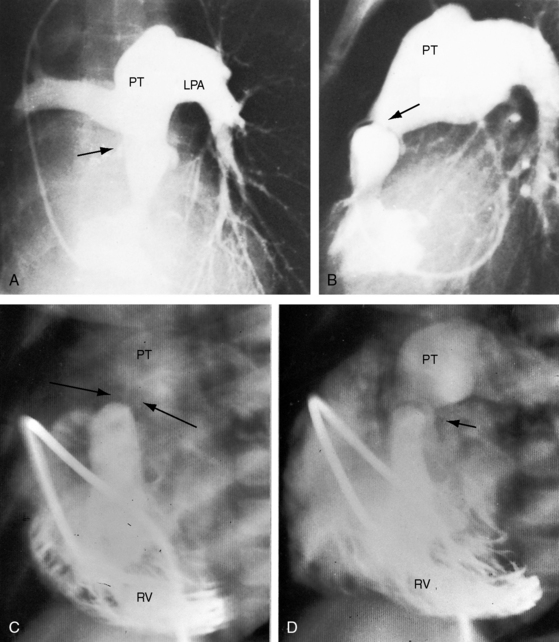
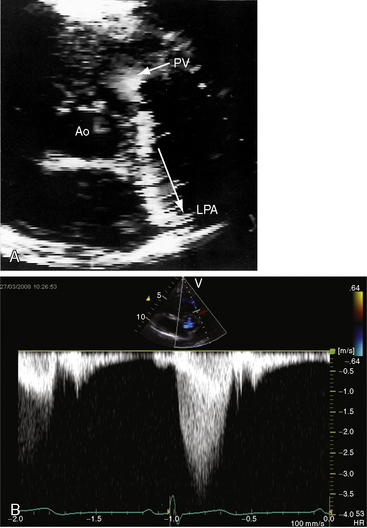
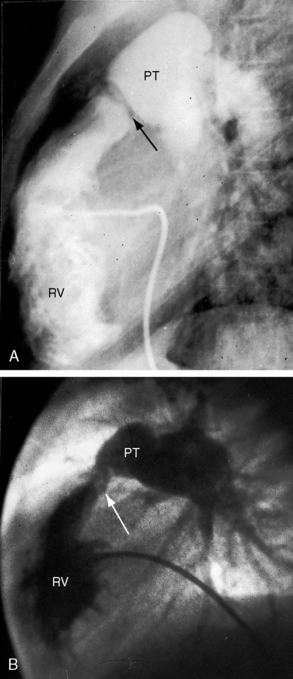
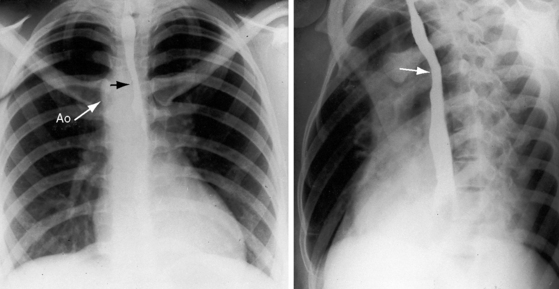
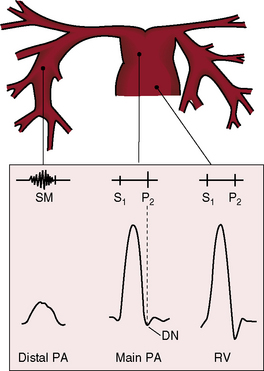
Congenital obstruction to right ventricular outflow in hearts with two noninverted ventricles originates in, below, or above the pulmonary valve. Three morphologic types of pulmonary stenosis involve the pulmonary valve: (1) typical mobile dome-shaped; (2) dysplastic; and (3) bicuspid. Dome-shaped pulmonary valve stenosis was described in 1761 by John Baptist Morgagni1 and is characterized by a thin mobile valve mechanism with a narrow central opening at its apex (Figure 11-1). Three rudimentary raphes extend from the central opening to the wall of the pulmonary artery, but separate leaflets and separate commissures cannot be identified. Pinpoint dome-shaped pulmonary valve stenosis in neonates is sometimes referred to as functional pulmonary atresia (see Figure 11-1A,B, lower). The pulmonary trunk is consistently dilated because of an inherent medial abnormality2 that is coupled with the mobile dome-shaped valve but not with its functional state (see Figure 11-1). The jet from the stenotic valve breaks up on striking the apex of the pulmonary trunk. The pressure component of total energy increases with a proportionate increase in pressure in the left branch (see Figure 11-35A).3 The physics of jet dispersion is believed to account for the larger size of the left branch. Calcification of a dome-shaped stenotic pulmonary valve is exceptional and is reserved for older patients (see section X-Ray).4 Dysplastic pulmonary valve stenosis is much less common and is characterized by myomatous thickening of three separate but poorly mobile leaflets without commissural fusion (Figure 11-2; Box 11-1).5,6 Bicuspid pulmonary valve stenosis is a feature of Fallot’s tetralogy (see Chapter 18). Isolated bicuspid pulmonary valves are rare and are of little or no functional significance.
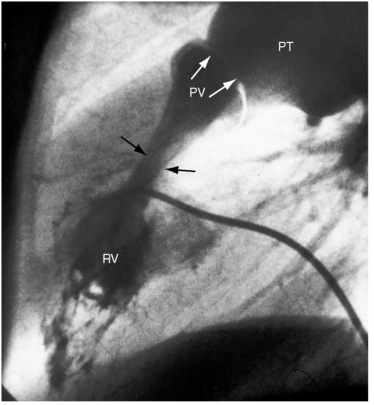
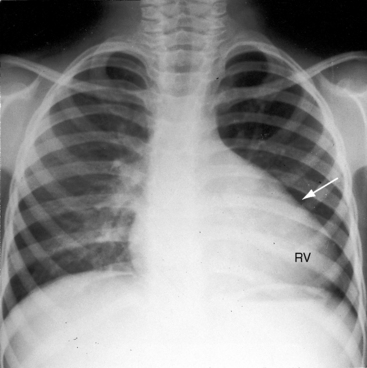
Subvalvular pulmonary stenosis can be infundibular or subinfundibular. The infundibular variety is caused by anterior and rightward deviation (malalignment) of the infundibular septum and is dealt with in Chapter 18. Secondary hypertrophic infundibular pulmonary stenosis accompanies—is secondary to—severe pulmonary valve stenosis (Figure 11-3 and see also Figure 11-38). Stenosis of the ostium of the infundibulum is a rare form of fixed obstruction to right ventricular outflow (see Figures 11-32 and 11-37). Subinfundibular stenosis or double-chambered right ventricle, described by Thomas Peacock7 in 1858, is a rare form of congenital obstruction to right ventricular outflow. Obstructing muscle bundles within the right ventricular cavity were the subject of Sir Arthur Keith’s8 Hunterian lecture in 1909. The double-chambered right ventricle is divided into a high-pressure inlet portion and a low-pressure outlet portion by normal or anomalous muscle bundles9,10 or by apical trabecular muscle sequestered from the rest of the right ventricle.11 The degree of obstruction varies from nil to severe to virtually complete.10 Double-chambered right ventricle usually coexists with a ventricular septal defect.9,10
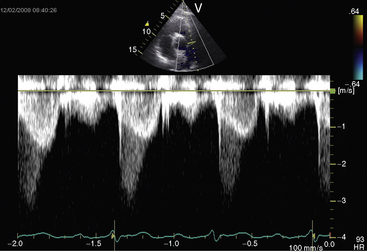
Figure 11-38 Continuous wave Doppler scan from a 32-year-old man with severe mobile dome-shaped pulmonary valve stenosis (PS) and secondary hypertrophic subpulmonary stenosis (compare with Figure 11-3). The asymmetric profile within the envelope of the continuous wave Doppler signal represents the gradient across the zone of secondary hypertrophic subpulmonary stenosis.
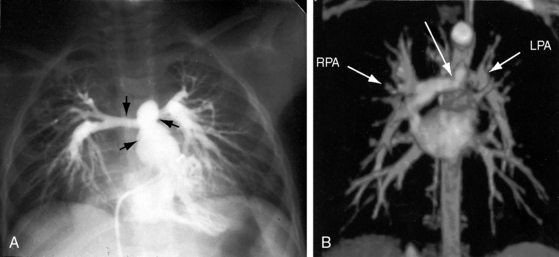
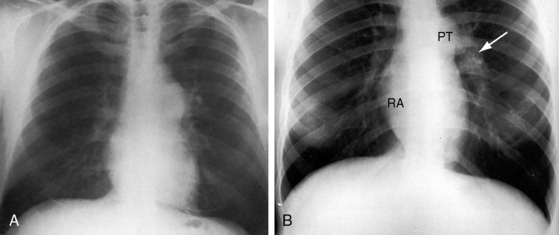
Pulmonary artery stenosis (supravalvular), described by Oppenheimer12 in 1938, is caused by narrowing of the pulmonary trunk, its bifurcation, or its primary or intrapulmonary branches (Figures 11-4 and 11-5).13 Stenosis of the pulmonary artery and its branches usually occurs as an isolated malformation13 and can be unilateral or bilateral, single or multiple, and segmental or tubular (see Figures 11-4 and 11-5).13 Intrapulmonary arteries distal to the stenoses tend to be dilated (see Figures 11-4 and 11-5). Rarely, a membranous form of obstruction occurs immediately above the valve.13
Neonates, especially premature, normally exhibit a disparity in size between the pulmonary trunk and its proximal branches. Angulations at the origins of the branches cause a drop in systolic pressure in the absence of morphologic pulmonary artery stenosis (see Chapter 2, Figure 2-4).14,15 The small pulmonary trunk and small proximal branches in Fallot’s tetralogy are discussed in Chapter 18. In Williams syndrome, bilateral stenosis of pulmonary artery branches is associated with supravalvular aortic stenosis (see Chapter 7).16 Experimental constriction of the pulmonary trunk in fetal lambs results in thin-walled intrapulmonary resistance vessels.17
In 1941, McAlister Gregg,18 an Australian ophthalmologist, described a relationship between maternal rubella and congenital abnormalities in offspring. What came to be known as the rubella syndrome consists of stenosis of the pulmonary artery and its branches with patent ductus arteriosis.19,20 Maternal rubella can have serious noncardiac effects that include spontaneous abortion, stillbirth, mental retardation, cataracts, and deafness. Viremia with placental and fetal infection during initial exposure is a prerequisite for the rubella syndrome. Fetal risk is small when infection occurs later than the 16th week of gestation.20 A worldwide rubella epidemic in 1962 was followed in 1964 and 1965 by an epidemic in the United States that affected approximately 12.5 million patients and caused 11,000 fetal deaths.19 Of an estimated 20,000 infants born with the rubella syndrome, approximately 2,100 died as neonates.19
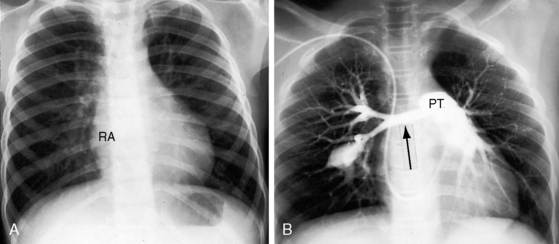
The physiologic consequences of pulmonary stenosis result from increased resistance to right ventricular discharge. Systolic pressure is elevated proximal to the obstruction and is normal or reduced distally. Pulmonary artery stenosis causes an increase in systolic pressure in the pulmonary trunk, which is proximal to the obstruction (see Figure 11-19). Valvular and subvalvular pulmonary stenosis increase the systolic pressure in the right ventricle, which is proximal to the obstruction. The gross morphologic response of the right ventricle to pressure overload is new sarcomeres in parallel, hence an increase in thickness of the free wall and ventricular septum, adaptive responses appropriate for developing power.21 Cavity size remains normal.22 In neonates with pinpoint pulmonary stenosis, cavity size is reduced.
The ultrastructural response to mechanical stress depends on myocyte maturity or immaturity at the time the inciting stimulus of overload becomes operative.21 In pulmonary stenosis, the inciting stimulus is present at birth when cardiomyocytes are immature and capable of replication, which is accompanied by capillary angiogenesis. Accordingly, each replicated normal-sized myocyte is paired with its own capillary, so the myocyte:capillary ratio (capillary density) is normal. However, a morphologic right ventricle is perfused by a morphologic right coronary artery, which imposes an inherent limitation.
Children and young adults with mild to moderate pulmonary valve stenosis have normal or near normal right ventricular function and can increase cardiac output with exercise.23 In severe pulmonary valve stenosis, however, stroke volume and cardiac output are fixed.24 Systolic contraction of hypertrophied infundibular muscle (see Figure 11-3) is reinforced by exercise.23
Pressure overload of the right ventricular can result in systolic and diastolic dysfunction of the left ventricle.25,26 Systolic dysfunction has been ascribed to chronic underfilling of the left ventricle, and diastolic dysfunction has been ascribed to displacement of the hypertrophied septum into the left ventricular cavity.
History
Typical mobile dome-shaped pulmonary valve stenosis is relatively common, with a prevalence rate as high as 10% of cases of congenital heart disease.27,28 Gender distribution is equal or with female prevalence.28 This is also the case in dysplastic pulmonary valve stenosis.29
The murmur of pulmonary stenosis is usually discovered at birth because the anatomic and physiologic conditions necessary for its production are present at birth. Parents are told that their newborn has congenital heart disease before discharge. However, the murmur of pinpoint pulmonary stenosis (see Figure 11-1, lower) is disarmingly soft. Murmurs at nonprecordial thoracic sites accompany pulmonary artery stenosis and in neonates and infants are easily overlooked or lost in the rapid breath sounds. A history of first trimester maternal rubella arouses suspicion of pulmonary artery stenosis.
Familial recurrence of isolated pulmonary valve stenosis is uncommon if not rare (Figure 11-6).30–33 In dysplastic pulmonary valve stenosis, the converse is the case.5 Some family members have a dysplastic valve, and others have a mobile dome-shaped valve.5 Similar observations have been made in the hereditary canine pulmonary valve dysplasia of beagles,34 in whom dysplastic pulmonary stenosis and dome-shaped pulmonary stenosis occur in litter mates.34 Familial Noonan’s syndrome frequently occurs with dysplastic pulmonary valve stenosis and has appeared in three generations.35,36 Successful pregnancy is possible in females with Noonan’s syndrome.37
Normal birth weights and normal growth and development are characteristic of mobile dome-shaped pulmonary valve stenosis. However, in Noonan’s syndrome with dysplastic pulmonary valve stenosis, growth and development are poor.5,29,38 Pulmonary artery stenosis is associated with low birth weights and retarded physical and mental development in the rubella syndrome and in Williams syndrome (see Chapter 7).19,39
Neonates with pinpoint pulmonary valve stenosis experience rapidly progressive cardiac failure and early death. However, most patients with mobile dome-shaped pulmonary valve stenosis experience little or no difficulty in infancy and childhood.40 In a review of 69 cases, the average age at death was 26 years; seven patients survived to age 50 years, and three survived to 70 and 75 years. In 21 adults, the average follow-up period was 50 years.41 Examples are found of survival into the sixth, seventh, and eighth decades,42–46 with one patient reaching 78 years.42 Longevity depends on three variables: 1, the initial severity of stenosis; 2, whether a given degree of stenosis remains constant or progresses; and 3, whether the function of the afterloaded right ventricle is preserved.24,40,47
The normal pulmonary valve orifice increases linearly with age and body surface area.48 The orifice of a stenotic mobile dome-shaped pulmonary valve increases with age, but not necessarily at the rate of somatic growth.40,48–50 Mild pulmonary valve stenosis in infancy usually remains mild,51 but moderate to severe pulmonary stenosis tends to progress.44 Stenosis of the pulmonary artery and its branches is not progressive. Fibrous thickening and occasionally calcification are responsible for increasing the degree of stenosis in older adults.
Equivalent degrees of pulmonary stenosis may handicap one patient in childhood but leave another relatively free of symptoms in adulthood. That mild pulmonary stenosis is asymptomatic is not surprising, but it is surprising that an appreciable number of patients with moderate to severe pulmonary stenosis claim to be virtually asymptomatic. Patients with right ventricular systolic pressures between 50 and 100 mm Hg include a New Zealand long-distance swimmer, a long-distance runner, and an English hockey captain.52 The author’s patients have included a 17-year-old boy who played baseball despite a right ventricular systolic pressure of nearly 200 mm Hg, a 32-year-old man who had run the quarter mile in high school despite a right ventricular systolic pressure of 75 mm Hg, and a woman with a right ventricular pressure of nearly 200 mm Hg who worked fulltime and had recurrent ascites for 7 years before death at age 60 years. Dyspnea and fatigue are mild as long as the right ventricle maintains a normal stroke volume at rest and augments its stroke volume with exercise.24,47 However, relatively asymptomatic patients can have rapid deterioration. Cardiac output is inadequate even at rest when the hemodynamic burden imposed on the right ventricle leads to right ventricular failure, which is the commonest cause of death.43
Giddiness and light-headedness with effort prefigure syncope.52 Children and adults occasionally experience the chest pain of right ventricular myocardial ischemia.46,53 A 3-year-old with severe pulmonary artery stenosis died during an episode of chest pain and at necropsy had infarction of the right ventricular free wall and interventricular septum.53 Sudden death has been associated with right ventricular infarction and an abnormal right coronary artery.54 Dilated thin-walled intrapulmonary artery aneurysms distal to the stenoses of pulmonary artery branches (see Figure 11-5B) are sources of hemoptyses that can be intermittent and mild or recurrent and brisk.13
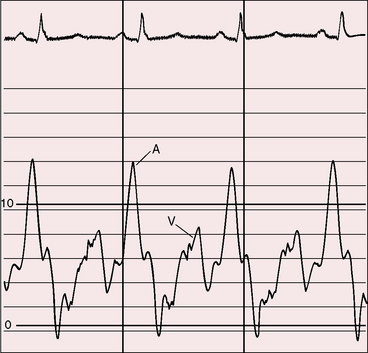
Severe pulmonary stenosis is accompanied by giant jugular venous A waves (Figure 11-7) of which patients are subjectively aware, especially during effort or excitement. These neck pulsations can be seen in the mirror when a young man shaves or when a young woman sits at her vanity. A 13-year-old girl with pulmonary valve stenosis and congenital complete heart block was aware of intermittent amplification of A waves as her right atrium randomly contracted against a closed tricuspid valve, and a 15-year-old boy was unpleasantly aware of intermittent amplification of jugular venous A waves caused by premature ventricular beats.
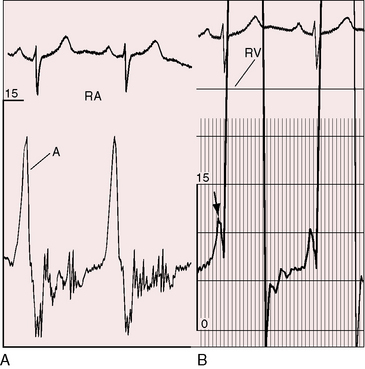
Figure 11-7 Pressure pulses from the 47-year-old woman with severe pulmonary valve stenosis referred to in Figure 11-1. A, Large right atrial (RA) A wave. B, Presystolic distention (arrow) of the right ventricle (RV) coincided with the large right atrial A wave.
Mobile dome-shaped pulmonary valve stenosis is a substrate for infective endocarditis44 that can induce a medical valvotomy when tissue is interrupted and orifice size increases.52 Jet lesions in pulmonary artery stenosis can serve as substrates for infective endarteritis.13
Physical appearance
Infants with mobile dome-shaped pulmonary valve stenosis occasionally have chubby, round, bloated facies with highly colored cheeks (Figure 11-8A) and well-developed fat deposits.52 The digits may be erythematous or frankly red in response to a small or intermittent right-to-left shunt through a patent foramen ovale (see Chapter 16).36,55
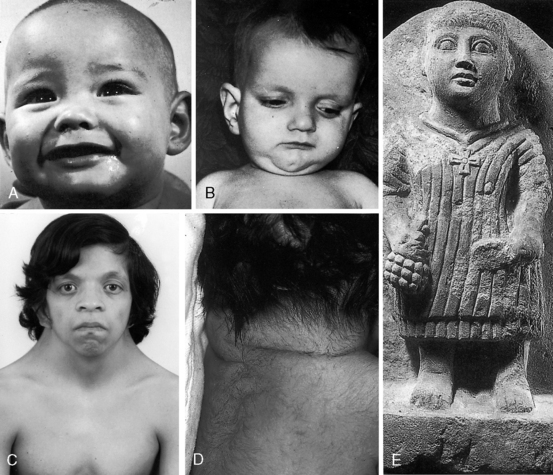
Noonan’s syndrome (Figure 11-8C)37,55,56 is characterized by short stature, webbed neck, pterygium colli, ptosis,hypertelorism, lymphedema, low-set ears, low anterior and posterior hairlines, flat or shield chest, pectus excavatum or carinatum, hyperelastic skin, inguinal hernia, nevi, dystrophic nails, micrognathia, hypospadias, and small undescended or cryptorchid testes.
About one third of patients with Noonan’s syndrome are mentally retarded, and approximately two thirds have congenital heart disease, especially dysplastic pulmonary valve stenosis (60%) and hypertrophic cardiomyopathy (20%).36,55,57–59
The rubella syndrome is characterized by cataracts, retinopathy, deafness, hypotonia, dermatoglyphic abnormalities, and mental retardation.60 Height and weight are usually normal for age despite intrauterine growth retardation. Stenosis of the pulmonary artery and its branches and patent ductus arteriosis (see Chapter 20) are the most frequent types of coexisting congenital heart disease.60
Williams syndrome includes a small chin, large mouth, patulous lips, blunt upturned nose, wide-set eyes, broad forehead, baggy cheeks, and malformed teeth (see Chapter 7). The most frequent coexisting congenital heart disease is pulmonary artery stenosis with supravalvular aortic stenosis (see Chapter 7).
Alagille syndrome, also called arteriohepatic dysplasia or the Alagille-Watson syndrome, is an autosomal dominant disorder with abnormalities of liver, eyes, kidneys, and skeleton.61,62 Facial appearance is characterized by a prominent overhanging forehead, deep-set eyes, and a small pointed chin (Figure 11-8B).61–63 The most frequent coexisting congenital heart disease is stenosis of the pulmonary artery and its branches.62
Cornelia de Lange’s syndrome expresses itself with low birth weight, slow growth, small stature, microcephaly, thin eyebrows that meet at midline, long eyelashes, short upturned nose, thin downturned lips, hirsutism, small hands and feet, incurved fifth fingers, cleft palate, and missing limbs or portions of limbs. The incidence rate of pulmonary valve stenosis is reportedly 39%.64
Pulmonary stenosis also occurs in LEOPARD syndrome, which is characterized by multiple lentigines and café-au-lait spots.65
Arterial pulse
When severe pulmonary stenosis is accompanied by right ventricular failure, especially with coexisting left ventricular dysfunction, the arterial pulse is reduced. Asymmetry of right and left brachial and carotid arterial pulses (right greater than left) is a feature of supravalvular aortic stenosis that may accompany stenosis of the pulmonary artery and its branches (see Chapter 7).
Jugular venous pulse
The jugular venous A wave is distinctive and increases progressively as the stenosis increases (Figures 11-8A and 11-9), culminating in a giant A wave that leaps to the eye, towering above and dwarfing the other waves of the venous pulse.27 Powerful right atrial contraction generates a giant A wave via the superior vena cava and a presystolic liver pulse via the inferior cava. With the advent of right ventricular failure and tricuspid regurgitation, the large A wave is accompanied by an increase in the V wave. The liver then manifests presystolic and systolic pulsations.
Precordial movement and palpation
The thrill associated with pulmonary valve stenosis is maximal in the second left intercostal space, with radiation upward and to the left because the intrapulmonary jet is directed upward and toward the left pulmonary artery (see Figure 11-35A).3 When secondary hypertrophic subpulmonary stenosis coexists, the thrill is maximal in the third or fourth left intercostal space (see Figure 11-3).
Palpation of the right ventricular impulse is best achieved with applying the fingertips between the ribs along the left sternal edge during full held exhalation or with applying a single fingertip against the diaphragm in the subzyphoid area.66,67 The subzyphoid technique is especially useful in infants because rapid respiratory excursions interfere with parasternal palpation.
The gentle right ventricular impulse of mild pulmonary valve stenosis is more readily palpated after exercise or in infants after the stress of feeding or crying. In severe pulmonary valve stenosis, the right ventricular impulse is forceful and sustained and is detected up to and including the third intercostal space.66–68 Infundibular pulmonary stenosis relegates the high-pressure zone to the inflow portion of the right ventricle, so the accompanying impulse is assigned to the fourth and fifth left intercostal spaces. Subinfundibular stenosis is accompanied by a deceptively unimpressive right ventricular impulse confined to the fifth interspace or subxyphoid area. The pulmonary trunk is necessarily distal to the stenosis and cannot be palpated despite conspicuous dilation.
A presystolic impulse along the lower left sternal border and in the subxyphoid area (see Figure 11-7B) indicates presystolic distention of the right ventricle in response to the increased force of right atrial contraction that is also responsible for the large jugular venous A wave (see Figure 11-7A). A prominent pulmonary ejection sound is occasionally palpable in the second left intercostal space during held exhalation.