Chapter 9 Congenital Obstruction to Left Atrial Flow
Mitral Stenosis, Cor Triatriatum, Pulmonary Vein Stenosis
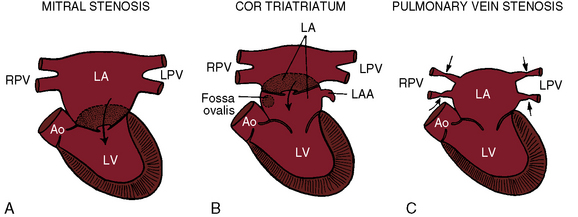
Congenital obstruction to left atrial flow can originate at or near the junction of the pulmonary veins and the left atrium (pulmonary vein stenosis), within the left atrium (cor triatriatum), immediately above the mitral valve (supravalvular stenosing ring), or within the mitral apparatus (mitral stenosis; Figure 9-1; Box 9-1). Pure or relatively pure forms of each defect are emphasized in this chapter, although a variety of anomalies often coexist.1–3 Pulmonary veno-occlusive disease is covered in Chapter 14, total anomalous pulmonary venous connection with obstruction is covered in Chapter 15, and hypoplastic left heart with a hypoplastic mitral orifice is covered in Chapter 31.
Box 9-1 Congenital Obstruction To Left Atrial Flow With A Functionally Adequate Left Ventricle
Congenital Mitral Stenosis
Congenital mitral stenosis
The incidence rate has been estimated at 0.6% of necropsy cases of congenital heart disease and 0.21% to 0.42% of clinical cases.4 Congenital mitral stenosis with a functionally adequate left ventricle includes the following malformations in approximate order of frequency (see Box 9-1):5–9
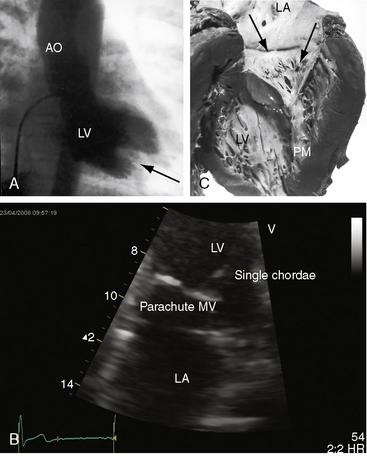
Flow across a normal mitral orifice is between the leaflets (interleaflet) and the chordae tendineae (interchordal). In a parachute mitral valve, all chordae tendineae insert into a single papillary muscle and the interchordal spaces are reduced or obliterated,10 so flow cannot be interchordal (see Figures 9-2B and 9-3).
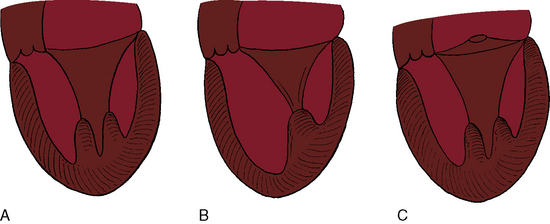
A parachute mitral valve is usually one of the components of Shone’s complex, a developmental combination of four obstructive lesions: namely, supravalvular stenosing ring, parachute mitral valve, subaortic stenosis, and coarctation of the aorta (Figures 9-3 and 9-10).1,2,15,16 All four lesions are not always present; if they are present, they may not be functionally significant.2,10,20 The supravalvular mitral ring can be rudimentary (see Figure 9-3C), or can bridge the mitral orifice as a stenosing diaphragm (see Figure 9-3B).8,9,18
History
There is a male predilection in congenital mitral stenosis,1 in contrast to rheumatic mitral stenosis, which has a female predilection. Familial recurrence has not been reported. If stenosis is severe, symptoms begin shortly after birth when pulmonary blood flow commences and suddenly enters the obstructed left atrium. Fifty percent of symptomatic infants die within 6 months, but an occasional infant is asymptomatic and a few remain relatively free of symptoms for years.21 With a parachute mitral valve or a supravalvular mitral ring, longevity is better (median, 10 years and 5.5 years, respectively), but short chordae tendineae and obliterated interchordal spaces result in death at a median age of 6 months.6,10 Anomalous mitral arcade or double-orifice mitral valve permits adult survival when the mitral apparatus functions normally or is purely regurgitant.5,14
Orthopnea, dyspnea, tachypnea, and paroxysmal cough are results of pulmonary edema that is punctuated by lower respiratory infections.1,21–23 Congenital mitral stenosis is occasionally associated with syncope21 but seldom with hemoptysis.23 Aphonia has been attributed to compression of the recurrent laryngeal nerve by a dilated hypertensive pulmonary trunk, analogous to hoarseness in adults with pulmonary hypertension and rheumatic mitral stenosis. Infective endocarditis is rare.1
Physical Appearance
Mild cyanosis coincides with congestive heart failure.21,22 Recurrent lower respiratory infections and the catabolic effects of heart failure account for physical underdevelopment.21,23
Arterial Pulse, Jugular Venous Pulse, Precordial Movement, and Palpation
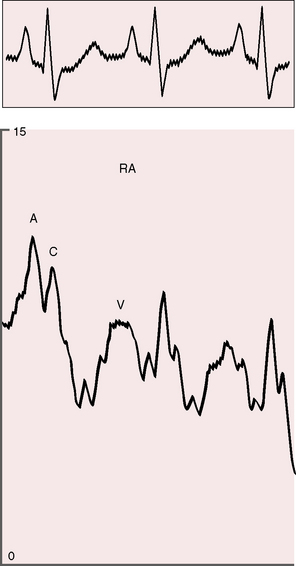
The arterial pulse is normal and confirms normal sinus rhythm, which is the rule in congenital mitral stenosis.21 The jugular venous pulse has an increased A wave because of pulmonary hypertension (Figure 9-4). A precordial bulge is common,23 and a right ventricular impulse is palpable atthe lower left sternal border and subxiphoid area.21,23
Auscultation
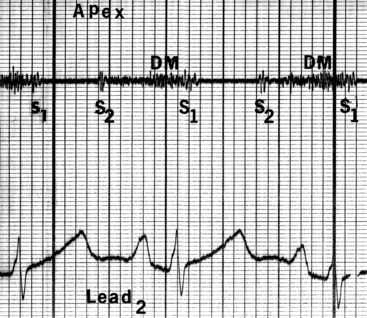
Neither a loud first heart sound nor an opening snap are heard; the necessary preconditions of these two auscultatory signs—abrupt opening and closing movements of the belly of a mobile anterior mitral leaflet—are not features of congenital mitral stenosis (Figures 9-5 and 9-6).1,10,11,23
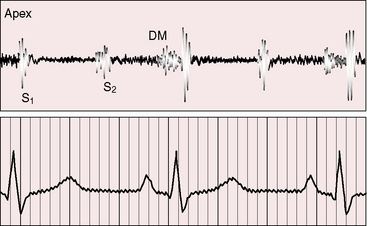
Figure 9-6 Phonocardiogram from the 3-year-old girl with congenital mitral stenosis referred to in Figure 9-4. The first heart sound (S1) is loud, but there is no opening snap. A soft mid-diastolic murmur (DM) is followed by presystolic accentuation.
In the presence of a parachute mitral valve or a supravalvular ring,17 a holosystolic murmur at the apex or lower left sternal edge is the result of mitral regurgitation or pulmonary hypertensive tricuspid regurgitation.22 An apical mid-diastolic murmur with presystolic accentuation (see Figures 9-5 and 9-6) is exceptional because the rapid heart rate in infants shortens diastole1,23 and because a dilated hypertensive right ventricle displaces the left ventricle from the apex.24,25 The pulmonary component of the second heart sound is loud because of pulmonary hypertension that sets the stage for the Graham Steell murmur of high-pressure pulmonary regurgitation.1,18
Electrocardiogram
Atrial fibrillation is exceptional in contrast to rheumatic mitral regurgitation.1 Pulmonary hypertension results in right atrial P wave abnormalities,1 right axis deviation,1 and right ventricular hypertrophy (Figure 9-7).1
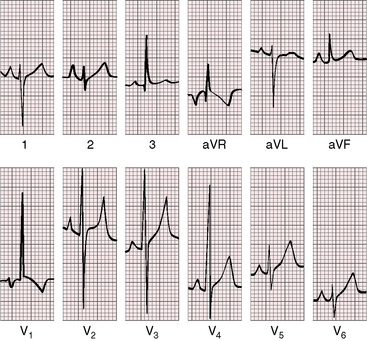
Figure 9-7 Electrocardiogram from the 2-year-old girl with Shone’s complex and pulmonary vascular disease referred to in Figure 9-5. Tall peaked right atrial P waves are present in leads 1, 2, and V2. There is marked right axis deviation. Right ventricular hypertrophy is manifested by the tall monophasic R wave in lead V1 and the deep S wave in lead V6. Large RS complexes in lead V2 and V3 suggest biventricular hypertrophy because of coarctation of the aorta.
X-Ray
Pulmonary venous congestion includes Kerley’s lines (Figure 9-8).1,23 Mild to moderate left atrial enlargement is recognized in the lateral projection (see Figure 9-8B). Straightening of the left cardiac border (Figure 9-9) by an enlarged left atrial appendage is much less common than in rheumatic mitral stenosis.1 Calcification of the mitral valve is absent in the x-ray and absent histologically.26 The pulmonary trunk, right ventricle, and right atrium are enlarged because of pulmonary hypertension (see Figures 9-8 and 9-9).
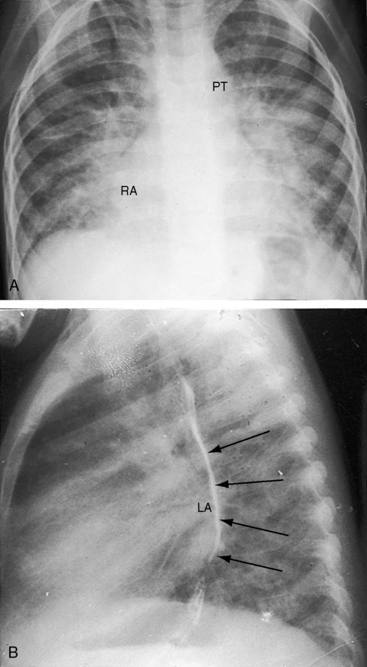
Figure 9-8 X-rays from the 2-year-old girl with Shone’s complex whose phonocardiogram is shown in Figure 9-5. A, Pulmonary venous congestion is striking. The pulmonary trunk (PT) and right atrium (RA) are dilated. B, The lateral film shows displacement of the barium esophagram by an enlarged left atrium (LA).
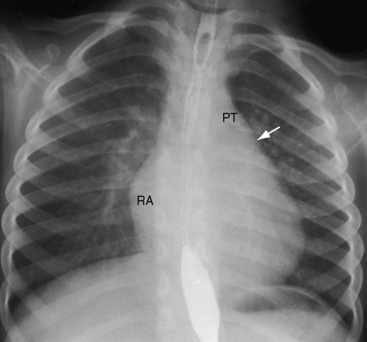
Figure 9-9 X-ray from the 3-year-old girl with congenital mitral stenosis referred to in Figures 9-4 and 9-6. There is bilateral hilar pulmonary venous congestion. The right atrium (RA) and pulmonary trunk (PT) are dilated, and the left cardiac border is straightened by an enlarged left atrial appendage (arrow).
Echocardiogram
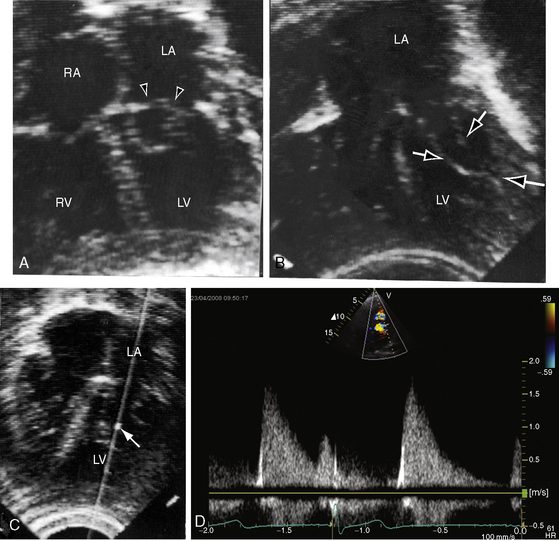
Congenital mitral stenosis is characterized by two well-formed papillary muscles with a reduced interpapillary distance.10 A parachute mitral valve is characterized by a single papillary muscle into which all chordae tendineae converge (Figures 9-3B,C and 9-10).16,27–29 The effective orifice size cannot be determined with two-dimensional echocardiography because flow is interchordal and the valve is eccentric (see Figure 9-10B), but Doppler scan interrogation can determine the gradient and the functional orifice size (see Figure 9-10C,D). A supravalvular ring is more readily identified when it is not attached to the mitral leaflets (see Figures 9-3B and 9-10A).6 Otherwise, the ring is imaged only in diastole.6 An anomalous mitral arcade has multiple papillary muscles with few or no chordae tendineae interposed between the arcade and the leaflets.27 The relative sizes of the two orifices of a double-orifice mitral valve can be determined, the chordal insertions can be characterized, and the functional state of the valve can be established as normal, stenotic, or incompetent.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree