Admixture lesions
The combination of cyanosis and increased pulmonary blood flow indicates an admixture lesion. In most cardiac malformations classified in this group, a single cardiac chamber receives the entire systemic and pulmonary venous blood flows as they return to the heart. These two blood flows mix and then the mixture leaves the heart into both the aorta and pulmonary artery. The admixture of blood can occur at any cardiac level: venous (e.g. total anomalous pulmonary venous connection), atrial (e.g. single atrium), ventricular (e.g. single ventricle), or great vessel (e.g. persistent truncus arteriosus).
Near-uniform mixing of the two venous returns occurs. Complete transposition of the great arteries is included in the admixture group because the patients are cyanotic with increased pulmonary blood flow. They have, however, only partial admixture of the two venous returns; this incomplete mixing leads to symptoms of severe hypoxia.
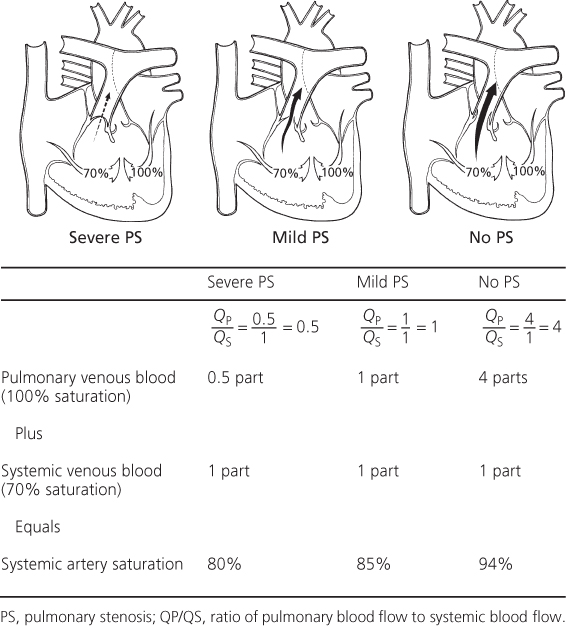
In an admixture lesion, the systemic arterial oxygen saturation is a valuable indicator of the volume of pulmonary blood flow, since the degree of cyanosis is inversely related to the volume of pulmonary blood flow.
In patients with large pulmonary blood flow, the degree of cyanosis is slight because large amounts of fully saturated blood return from the lungs and mix with a relatively smaller volume of systemic venous return (Figure 6.1). If the patient develops pulmonary vascular disease or pulmonary stenosis that limits pulmonary blood flow, the amount of fully oxygenated blood returning from the lungs and mixing with the systemic venous return is reduced, so the patient becomes more cyanotic and the hemoglobin and hematocrit values rise.
Figure 6.1 Estimation of the pulmonary blood flow in admixture lesions. Using a single ventricle, three clinical examples are shown, each with different degrees of pulmonary stenosis and pulmonary blood flow. Cyanosis is inversely related to the pulmonary blood flow. Assuming healthy lungs and complete mixture of the systemic and pulmonary venous return, the systemic arterial oxygen saturation represents the average of the contribution of the pulmonary blood flow (QP), represented by the pulmonary venous return, and the systemic blood flow (QS), represented by the systemic venous return. QP/QS can be estimated from the pulse oximetry value. PS, pulmonary stenosis; QP/QS, ratio of pulmonary blood flow to systemic blood flow.
Complete transposition of the great arteries (d-TGA or d-TGV)
This is the most frequently occurring condition with cyanosis and increased pulmonary blood flow.
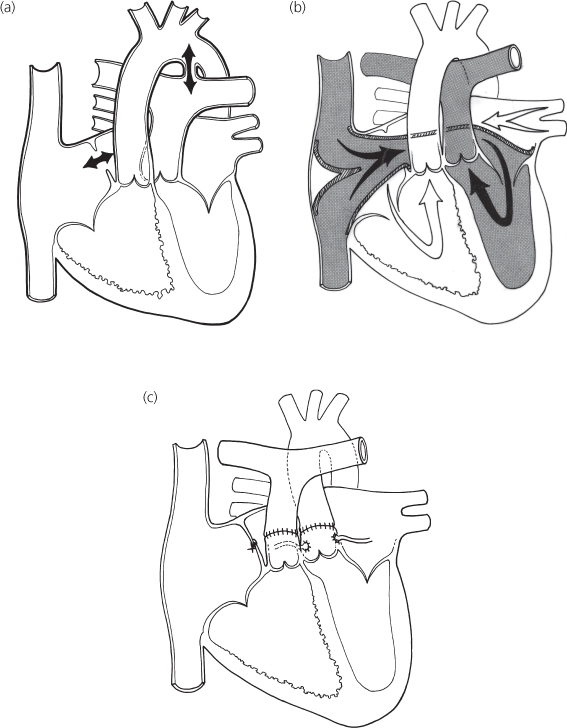
The term transposition indicates an anatomic reversal in anteroposterior, not left–right relationships. Normally, the pulmonary artery lies anterior to and slightly to the left of the aorta. In complete transposition of the great arteries (Figure 6.2a), the aorta lies anterior to the pulmonary artery. Normally, the anterior blood vessel arises from the infundibulum, which is the conus portion of the right ventricle. The aorta in complete transposition arises from the infundibulum of the right ventricle. The pulmonary trunk, on the other hand, originates posteriorly from the left ventricle.
Figure 6.2 Complete transposition of the great vessels (d-TGV). (a) Central circulation. Surgical options: (b) venous switch; (c) arterial switch.
Because of the transposition of the great arteries and their anomalous relationship to the ventricles, two independent circulations exist. The systemic venous blood returns to the right atrium, enters the right ventricle, and is ejected into the aorta, while the pulmonary venous blood flows through the left side of the heart into the pulmonary artery and returns to the lungs.
A communication must exist between the left and right sides of the heart to allow bidirectional shunting between of these two venous returns. The communication exists in one or more of the following: patent foramen ovale, atrial septal defect, ventricular septal defect, or patent ductus arteriosus. In about 60% of the patients, the ventricular septum is intact and the shunt occurs at the atrial level. In the other 40%, a ventricular septal defect is present. Pulmonary stenosis, often valvar and subpulmonic, may coexist.
In patients with an intact ventricular septum, the communication (either a patent foramen ovale or a patent ductus arteriosus) between the two sides of the circulation is often small. As these communications follow the normal neonatal course and close, neonates with transposition and an intact septum develop profound cyanosis. Because a greater degree of mixing usually occurs in patients with a coexistent ventricular septal defect, cyanosis is mild in such infants with transposition and diagnosis is sometimes delayed.
History
Complete transposition of the great arteries occurs more frequently in males. Cyanosis becomes evident shortly after birth. Without intervention, almost all infants exhibit dyspnea and other signs of cardiac failure in the first month of life; infants with intact ventricular septum develop cardiac symptoms in the first 2 days of life and are more intensely cyanotic than those with coexistent ventricular septal defect. In the absence of operation, death occurs, usually in neonates, and in nearly every patient by 6 months of age. Patients with ventricular septal defect and pulmonary stenosis are often the least symptomatic because the pulmonary stenosis prevents excessive pulmonary blood flow and enhances the flow of fully saturated blood through the ventricular septal defect into the aorta; these patients resemble those with tetralogy of Fallot.
Physical examination
Infants may be large for gestational age. Setting aside cyanosis and congestive cardiac failure, physical findings vary with the coexistent defect associated with the complete transposition. Neonates on the first day of life are often asymptomatic, except for cyanosis, but quickly develop tachypnea.
With an intact ventricular septum and an atrial shunt, either no murmur or a soft, nonspecific murmur is present. With an associated ventricular septal defect, a louder murmur is present. The second heart sound is single and loud along the upper left sternal border, representing closure of the anteriorly placed aortic valve. Although the murmur does not diagnose complete transposition, it can indicate the type of associated defect. If pulmonary stenosis coexists, the murmur often radiates to the right side of the back.
Electrocardiogram
Since the aorta arises from the right ventricle, its pressure is elevated to systemic levels and is associated with a thick-walled right ventricle. The electrocardiogram reflects this by a pattern of right-axis deviation and right ventricular hypertrophy. The latter is manifested by tall R waves in the right precordial leads. Right atrial enlargement is also possible. In neonates it may be indistinguishable from normal for the age.
Patients with a large volume of pulmonary blood flow, as with coexistent ventricular septal defect, also may have left ventricular enlargement/hypertrophy because of the volume load on the left ventricle.
Chest X-ray
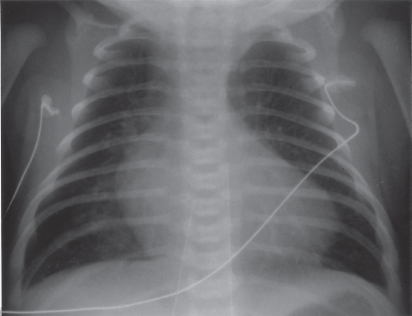
Cardiomegaly is generally present. The cardiac silhouette has a characteristic egg-shaped appearance (Figure 6.3); the superior mediastinum is narrow because the great vessels lie one in front of the other; the thymus is usually small. Left atrial enlargement exists in the unoperated patient.
Figure 6.3 Chest X-ray in complete transposition of the great vessels: cardiomegaly, narrow mediastinum, and increased pulmonary vasculature.
Echocardiogram
The key to the echocardiographic diagnosis of complete transposition is the recognition of an anteriorly arising aorta and a posteriorly arising pulmonary artery. In views parallel to the long axis of the left ventricle, both arteries course parallel to each other for a short distance. This appearance is not seen in a normal heart, where the great arteries cross each other at an acute angle. In views profiling the short axis of the left ventricle, the aorta is seen arising anterior and rightward of the central and posterior pulmonary artery (hence the term d-transposition, or dextro-transposition). A cross-sectional view of the aortic root allows demonstration of the origins, branching, and proximal courses of the coronary arteries.
In neonates with transposition, the interventricular septum usually has a flat contour when viewed in cross-section; however, as the infant ages, the septum gradually bows away from the right (systemic) ventricle and bulges into the left (pulmonary) ventricle.
Ventricular septal defect represents the most important associated lesion diagnosed by echocardiography; the shunt through it and any atrial septal defect or ductus is bidirectional, consistent with the physiology of transposition described earlier. The atrial septal defect may be small and restrictive (Doppler signals are high velocity) before balloon septostomy; after, it is typically large and unrestrictive, with a mobile flap of the torn fossa ovalis waving to and fro across the defect. Balloon septostomy may be performed under echocardiographic guidance.
Cardiac catheterization
Since echocardiography shows the diagnosis, the primary purpose of cardiac catheterization is the performance of interventional creation of an atrial septal defect (Rashkind procedure). In patients with an intact septum, oximetry data show little increase in oxygen saturation values through the right side of the heart, and little decrease through the left side. Among those with coexistent ventricular septal defect, larger changes in oxygen values are found. The oxygen saturation values in the pulmonary artery are higher than those in the aorta, a finding virtually diagnostic of transposition of the great arteries.
In all patients, right ventricular systolic pressure is elevated. When the ventricular septum is intact, the left ventricular pressure may be low; but in most patients with coexistent ventricular septal defect or in those with a large patent ductus arteriosus, the left ventricular pressure is elevated and equals that of the right (systemic) ventricle.
Angiography confirms the diagnosis by showing the aorta arising from the right ventricle and the pulmonary artery arising from the left ventricle, and it identifies coexistent malformations. Aortic root injection demonstrates coronary artery anatomy in preparation for surgery. A left ventricular injection is indicated to demonstrate ventricular septal defect(s) and pulmonic stenosis.
Palliative procedures
Hypoxia, one of the major symptom of infants with transposition of the great vessels, results from inadequate mixing of the two venous returns, and palliation is directed towards improvement of mixing by two means. Unless hypoxia is treated, it becomes severe, leading to metabolic acidosis and death.
Intravenous prostaglandin
This substance opens and/or maintains patency of the ductus arteriosus and improves blood flow from aorta to pulmonary artery.
Rashkind balloon atrial septostomy procedure
Patients with inadequate mixing benefit from the creation of an atrial septal defect (enlargement of the foramen ovale). At cardiac catheterization or by echocardiographic guidance, a balloon catheter is inserted through a systemic vein and advanced into the left atrium through the foramen ovale. The balloon is inflated and then rapidly and forcefully withdrawn across the septum, creating a larger defect and often improving the hypoxia.
Infants who do not experience adequate improvement of cyanosis despite a large atrial defect and patent ductus are rare. Factors responsible in these neonates include nearly identical ventricular compliances, which limits mixing through the atrial defect, and elevated pulmonary vascular resistance, which limits the ductal shunt and pulmonary blood flow. Increased intravenous fluids may benefit the patient by increasing blood volume.
Rarely, an atrial defect is created surgically by atrial septectomy, an open-heart procedure. A closed-heart technique, the Blalock–Hanlon procedure, was used previously, but frequently resulted in scarring of the pulmonary veins.
Corrective operation
Atrial (venous) switch (see Figure 6.2)
The first successful corrective procedure was performed by Senning in the 1950s and later modified by Mustard. These procedures invoke the principle that two negatives make a positive. Since the circulation of transposition is reversed at the arterial level, these operations reverse it the atrial level. This procedure involves removal of the atrial septum and creation of an intra-atrial baffle to divert the systemic venous return into the left ventricle and thus to the lungs, whereas the pulmonary venous return is directed to the right ventricle and thus to the aorta.
It can be performed at low risk in patients with an intact ventricular septum and at a higher risk in patients with ventricular septal defect. Serious complications, stroke, or death can occur in infants before an atrial (venous) switch procedure, which is usually done after 3–6 months of age.
The long-term results of the atrial switch procedure have been identified. Arrhythmias, the most frequent long-term complication, are often related to abnormalities of the sinoatrial node and of the atrial surgical scar. Sometimes these are life threatening, although the exact mechanism of sudden death in the rare child who succumbs is not usually known. Scarring can also cause systemic or pulmonary obstruction of the venous return. The most common significant complication is not sudden death but progressive dysfunction of the right ventricle, leading to death from chronic heart failure in adulthood. This complication is related to the right ventricle functioning as the systemic ventricle. Predicting which patients will develop failure and at the age postoperatively is not possible.
Arterial switch (Jatene) (see Figure 6.2c)
This operation, developed in the 1970s, avoids the complications inherent with the atrial (venous) switch and involves switching the aorta and pulmonary artery to the correct ventricle. The great vessels are transected and reanastomosed, so blood flows from left ventricle to aorta and from right ventricle to pulmonary arteries. Since the coronary arteries arise from the aortic root, they are transferred to the pulmonary (neoaortic) root. Certain variations of coronary artery origins or branching make transfer more risky. The arterial switch operation must occur early in life (within the first 2 weeks) before the pulmonary resistance falls and the left ventricle becomes “deconditioned” to eject the systemic pressure load.
Arterial switch is not free from complications: coronary artery compromise may result in left ventricular infarct or failure; pulmonary artery stenosis can result from stretching or kinking during the surgical repositioning of the great vessels; and the operative mortality may be higher, partly because of the risks of neonatal open-heart surgery.
The short- and long-term outcomes favor those receiving the arterial switch procedure.
Total anomalous pulmonary venous connection (TAPVC or TAPVR) (see Figure 6.4)
The pulmonary veins, instead of entering the left atrium, connect with a systemic venous channel that delivers pulmonary venous blood to the right atrium. Developmentally, this anomaly results from failure of incorporation of the pulmonary veins into the left atrium, so that the pulmonary venous system retains earlier embryologic communications to the systemic venous system.
In the embryo, the pulmonary veins communicate with both the left and right anterior cardinal veins and the umbilical vitelline system, both precursors of systemic veins. If the pulmonary veins, which form with the lungs as outpouchings of the foregut, are not incorporated into the left atrium, the result is anomalous pulmonary venous connection to one of the following structures: right superior vena cava (right anterior cardinal vein), left superior vena cava (distal left anterior cardinal vein), coronary sinus (proximal left anterior cardinal vein), or infradiaphragmatic site (umbilical–vitelline system), usually a tributary of the portal system.
Therefore, the right atrium receives not only the entire systemic venous return, but also the entire pulmonary venous return. The left atrium has no direct venous supply. An obligatory right-to-left shunt exists at the atrial level through either a patent foramen ovale or usually an atrial septal defect.
The volume of blood shunted from the right to the left atrium and the volume of blood that enters each ventricle depends upon their relative compliances. Ventricular compliance is influenced by ventricular pressures and vascular resistances. Right ventricular compliance normally increases following birth as pulmonary vascular resistance and pulmonary arterial pressure fall. Therefore, in most patients with total anomalous pulmonary venous connection, pulmonary blood flow becomes considerably greater than normal; systemic blood flow is usually normal. Since a disparity exists between the volume of blood being carried by the right and left sides of the heart, the right side becomes dilated and hypertrophied, whereas the left side is relatively smaller but near-normal size.
In patients with total anomalous pulmonary venous connection, the degree of cyanosis inversely relates to the volume of pulmonary blood flow. As the volume of pulmonary blood flow becomes larger, the proportion of the pulmonary venous blood to total venous blood returning to the right atrium becomes greater. As a result, the saturation of blood shunted to the left side of the heart is higher, being only slightly reduced from normal.
On the other hand, in hemodynamic situations in which the resistance to flow through the lungs is increased (e.g. the neonatal period), the volume of blood flow through the lungs is nearly normal (i.e. equal to systemic blood flow). Therefore, the pulmonary and systemic venous systems contribute nearly equal volumes of blood to the right atrium, and these neonates exhibit noticeable cyanosis.
Total anomalous pulmonary venous connection is an example of bidirectional shunting: a left-to-right shunt at the venous level and a right-to-left shunt at the atrial level since all the pulmonary venous blood returns to the right atrium.
Total anomalous pulmonary venous connection presents two clinical pictures. One resembles atrial septal defect and has no obstruction to the venous channel. The other shows intense cyanosis and a radiographic pattern of pulmonary venous obstruction. In this form, the connecting venous channel is narrowed and obstructed. These two are discussed separately in the following.
Figure 6.4 Total anomalous pulmonary venous connection. (a) Central circulation and surgical repair of unobstructed type; (b) central circulation in obstructed type.
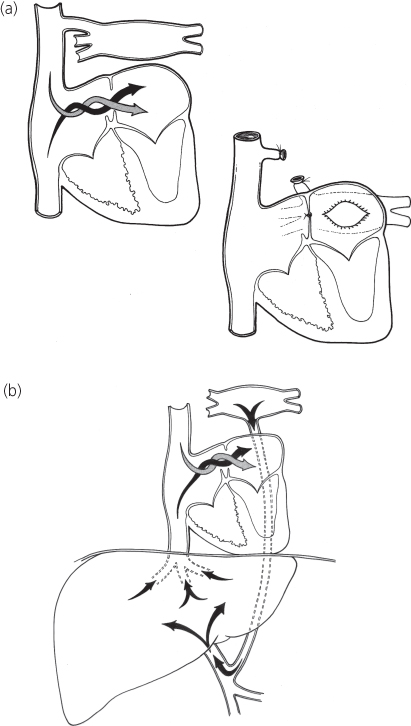
Total anomalous pulmonary venous connection without obstruction (see Figure 6.4a)
History
The clinical manifestations vary considerably. Usually, the anomaly is recognized in the neonatal period or with fetal echocardiography. If not operated upon in early infancy, most patients develop congestive cardiac failure, grow slowly, and have frequent respiratory infections, but a few may be asymptomatic into later childhood.
Physical examination
The degree of cyanosis varies because of differences in the volume of pulmonary blood flow. Although systemic arterial desaturation is always present, children with greatly increased pulmonary blood flow appear acyanotic or show only slight cyanosis.
The physical findings mimic isolated atrial septal defect. Cardiomegaly, precordial bulge, and right ventricular heave are found in older unoperated infants. A grade 2/6–3/6 pulmonary systolic ejection murmur due to excess flow across the pulmonary valve is present along the upper left sternal border. Wide, fixed splitting of the second heart sound is heard and the pulmonary component may be accentuated, reflecting elevated pulmonary pressure. A mid-diastolic murmur caused by increased blood flow across the tricuspid valve is found along the lower left sternal border and is associated with greatly increased pulmonary blood flow. In total anomalous pulmonary venous connection to the superior vena cava, a venous hum may exist along the upper right sternal border because of the large venous blood flow.
Electrocardiogram
The electrocardiogram reveals enlargement of the right-sided cardiac chambers with right-axis deviation, right atrial enlargement, and right ventricular enlargement/hypertrophy. Usually, the pattern reflecting volume overload, is an rSR′ pattern in lead V1.
Chest X-ray
Chest X-ray findings also resemble isolated atrial septal defect. Cardiomegaly, primarily of right-sided chambers, and increased pulmonary blood flow are found. In contrast to most other admixture lesions, the left atrium is not enlarged because blood flow through this chamber is normal.
Except for total anomalous pulmonary venous connection to a left superior vena cava (“vertical vein”), the roentgenographic contour is not characteristic. In this form, the cardiac silhouette can be described as a figure-of-eight or as a “snowman heart” (Figure 6.5a). The upper portion of the cardiac contour is formed by the enlarged left and right superior venae cavae. The lower portion of the contour is formed by the cardiac chambers.
Figure 6.5 Chest X-ray in total anomalous pulmonary venous connection. (a) Unobstructed (supracardiac) connection to the left superior vena cava (“snowman” heart). Upper portion of cardiac silhouette formed by dilated right and left superior venae cavae. (b) Obstructed (infradiaphragmatic) type. Pulmonary vascular congestion, a pleural effusion, and a small heart shadow.
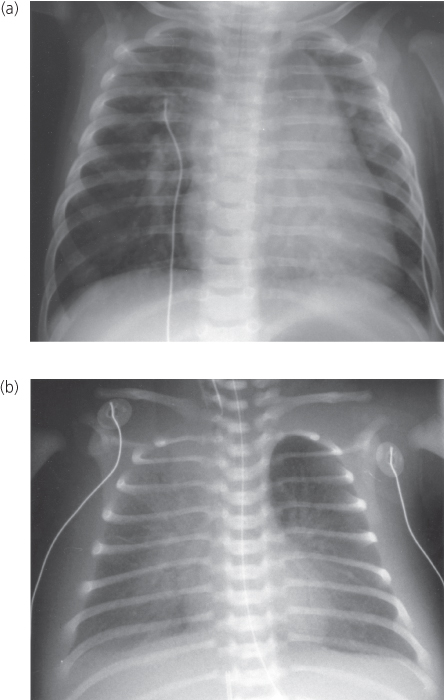
Echocardiogram
Cross-sectional echocardiography reveals an atrial septal defect and enlarged right atrium, right ventricle, and pulmonary arteries. The left atrium and left ventricle appear smaller than normal. In contrast to most normal neonates, with an atrial septal defect the shunt is from right atrium to left atrium. Doppler demonstrates a right-to-left atrial septal defect shunt because the only blood entering the left atrium is through the atrial septal defect. The individual pulmonary veins are visualized as they join a common pulmonary vein, which then connects to the coronary sinus, the superior vena cava by way of a vertical vein (the left-sided superior vena cava), or the hepatic portal venous system after a descent into the abdomen.
Cardiac catheterization
Oxygen saturation values in each cardiac chamber and in both great vessels are virtually identical. An increase in oxygen saturation is found in the vena cava, coronary sinus, or other systemic venous sites into which the pulmonary venous blood flows. The saturation of blood in the left atrium and left ventricle is reduced because of the obligatory right-to-left atrial shunt.
Pulmonary hypertension may be found in infants, but some patients, particularly older ones, show near-normal levels of pulmonary arterial pressures.
Pulmonary angiography is indicated. During the later phases of the angiogram (the so-called levophase), the pulmonary veins opacify and subsequently fill the connecting venous channel, delineating the anatomic form of anomalous pulmonary venous connection.
Operative considerations
Under cardiopulmonary bypass, the confluence of pulmonary veins, which lies directly behind the left atrium, is opened and connected to it (Figure 6.4a). The atrial communication is closed, and the connecting vessel is divided. This operation can be performed with low risk, even in neonates and younger infants.
Total anomalous pulmonary venous connection with obstruction (see Figure 6.4b)
In total anomalous pulmonary venous connection, an obstruction can be present in the channel returning pulmonary venous blood to the right side of the heart. Obstruction is always present in patients with an infradiaphragmatic connection and occasionally in patients with a supradiaphragmatic connection. In the latter, obstruction may occur intrinsically from narrowing of the channel or extrinsically if the channel passes between the bronchus and the ipsilateral branch pulmonary artery.
In infradiaphragmatic connection, four mechanisms contribute to obstruction in pulmonary venous flow: (1) the venous channel is long; (2) the channel traverses the diaphragm through the esophageal hiatus and is compressed by either esophageal or diaphragmatic action; (3) the channel narrows at its junction with the portal venous system; and (4) the pulmonary venous blood must traverse the hepatic capillary system before returning to the right atrium by way of the hepatic veins.
The obstruction elevates pulmonary venous pressure. Consequently, pulmonary capillary pressure is raised, leading to pulmonary edema and a dilated pulmonary lymphatic system. Pulmonary arterial pressure is elevated because of both elevated pulmonary capillary pressure and reflex pulmonary vasoconstriction. Because of the pulmonary hypertension, the right ventricle remains thick walled, does not undergo its normal evolution following birth, and remains relatively noncompliant. As a result, the volume of flow into the right ventricle is limited. Because of the reduced pulmonary blood flow, the patients show more intense cyanosis than those with without pulmonary venous obstruction.
The clinical features of total anomalous pulmonary venous connection with obstruction relate to the consequences of pulmonary venous obstruction and to the limited pulmonary blood flow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


