Chapter 32 Congenital Anomalies of the Coronary Circulation
The word coronary is derived from the Latin coronarius (pertaining to a crown), translated from the Greek stephanos (wreath), which refers to the crown-like or wreath-like arrangement of arteries that encircle the heart.1 This chapter deals with the coronary circulation—arterial and venous—including morphogenesis, the normal coronary arteries, and the congenital anomalies as classified in Table 32-1.
Table 32-1 Classification of Congenital Anomalies of the Coronary Circulation
| Congenital anomalies of coronary arteries unassociated with congenital heart disease |
| Congenital anomalies of coronary arteries associated with congenital heart disease |
| Acquired anomalies of coronary arteries resulting from congenital heart disease |
| Congenital anomalies of the coronary venous circulation |
In 1513, Leonardo da Vinci’s anatomic drawings of a bullock’s heart identified the coronary arteries (see Figure 32-3A) and the great cardiac vein (coronary sinus).2 Leonardo called attention to two vessels that arise from two external openings (aortic sinuses). In 1543, 500 years later, the circulation in the normal heart was characterized,3 and congenital anomalies of the coronary arteries were recognized.4 In 1543, Andreas Vesalius, the celebrated 16th century Flemish anatomist, depicted an anomalous right coronary artery originating from the left aortic sinus and passing anterior to the right ventricular outflow tract (De Humani Corporis Fabrica).
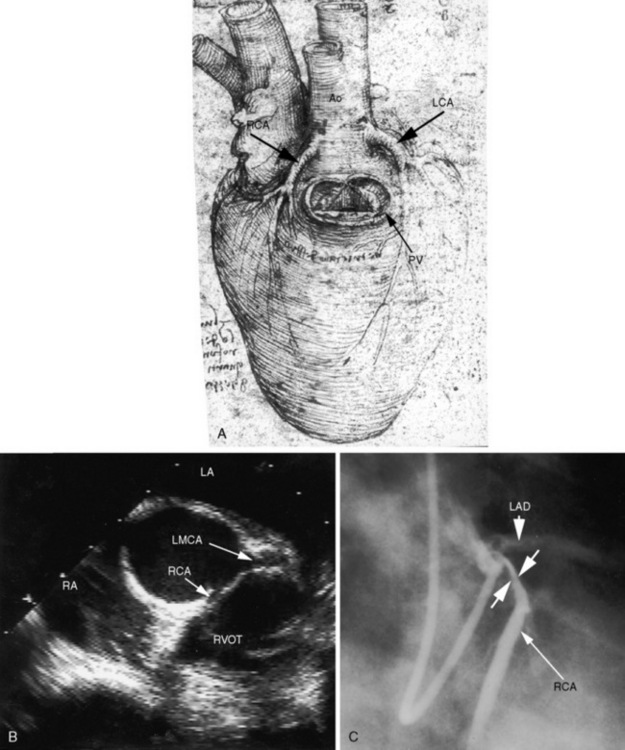
Figure 32-3 A, Drawing of a bullock’s heart by Leonardo da Vinci, circa 1513, with labels by the author. “The heart from the right side. The pulmonary artery has been removed to expose the pulmonary orifice and the semilunar valves guarding it. From the aorta spring the right and left coronary arteries.” B, Echocardiogram (short-axis view) from an asymptomatic 23-year-old man in whom the right coronary artery (RCA) originated from the left aortic sinus and passed between the aorta and right ventricular outflow tract. The left main coronary artery (LCA) originated from the left aortic sinus by a separate ostium and divided into the left anterior descending and circumflex coronary arteries (compare with Figure 32-1D, second illustration). (Ao = aorta; LAD = left anterior descending.) C, Coronary arteriogram from a 29-year-old woman with angina pectoris. The right coronary artery (RCA) originated from the left aortic sinus and was compressed (paired arrows) as it passed between the aorta and right ventricular outflow tract (compare with Figure 32-1D, second illustration). The left coronary artery originated by a separate ostium from the left aortic sinus and divided into the left anterior descending (LAD) and circumflex coronary arteries.
Selective coronary angiography was introduced in 1962 by Mason Sones5 and was a major step forward in imaging coronary artery anatomy in the living beating human heart. Techniques continue to advance and include transesophageal echocardiography, magnetic resonance imaging, computerized axial tomography, electron beam tomography, and three-dimensional reconstruction.6–8 Concurrently, nomenclature has evolved.9
Myocardial morphogenesis begins with the emergence of trabeculations in the luminal ventricular layers that permit an increase mass in the absence of a coronary circulation.10 Cardiac jelly in the embryonic luminal layer is the primitive site of metabolic exchange between cardiac mesenchyma and blood in the ventricular cavity.4,11 The human heart begins to beat as early as the 22nd day after conception, and a few days later, an ebb and flow circulation permits metabolic exchange.
The sequence of development of the coronary vascular bed begins with blood islands and coronary venous connections followed by coronary artery–to–aortic connections. Blood islands are epicardial layers of endothelial cells distended by nucleated erythrocytes. The blood islands proliferate, coalesce, and form rudimentary networks of vascular channels with no discernible connections to other islands or to the cavity of the ventricle. The second stage of development is a venous connection between the network of vascular channels and the coronary sinus, an arrangement that drains the blood islands and reduces their size. The distal coronary bed remains a loose intermingling vascular network until myocardial mass develops. In the third stage of development, the coronary arteries join the aorta through an arterial connection to the plexus of vascular channels. The third stage is established after aortopulmonary septation and after formation of the semilunar valves. Flow commences from the aorta into the proximal coronary arteries through myocardial capillaries, then into coronary veins and into the coronary sinus. It is unknown why the two coronary arteries originate from the right and left aortic sinuses that face the right ventricular outflow tract (Figure 32-1A). The embryonic great arteries contain six sinuses, three in the aorta and three in the pulmonary trunk. The endothelial outgrowths or anlagen (buds, sprouts) in the third aortic sinus and in all three pulmonary sinuses undergo rapid involution or do not develop. Aortic–to–coronary artery connections become evident before the appearance of precursor connections in the aortic sinuses. It has been speculated that the aortic sinus sites of the two coronary ostia are determined by mural tension that is increased by the catenoid configuration of the right and left sinuses, so that endothelium penetrates the aortic wall preferentially in the right and left sinus sites, establishing connections with the epicardial coronary plexus.
The capillary bed interposed between the coronary arterial and coronary venous circulations plays a pivotal role in the transport of oxygen and nutrients to the myocardium. Capillary density, defined as the ratio of the number of capillaries to the number of cardiomyocytes, is basic to the metabolic exchange between blood and tissues. Capillary density depends on angiogenesis—the capacity of capillaries to replicate. Fetal capillaries replicate in response to the hypoxic intrauterine environment. Postnatal angiogenesis is a diminishing continuation of that response.11
The coronary circulation includes three separate systems of veins.12 The largest system terminates in the coronary sinus and drains blood from most of the left ventricle. A second venous system drains most of the blood from the right ventricle. Adam Christian Thebesius, a German anatomist (1686-1732), described a third venous system consisting of tributaries that drain directly into the cardiac chambers.12 Before the discovery of the pulmonary circulation, Thebesian veins were considered pathways of blood flow from right heart to left heart.
The round or ovoid ostia of the two coronary arteries are located in the right and left anterior aortic sinuses, which are therefore called the coronary sinuses.1 The posterior aortic sinus is devoid of a coronary ostium and is appropriately called the noncoronary sinus.1 Normal coronary artery ostia are located in the middle of an aortic sinus just below the sinotubular junction and just above the upper margin of the aortic cusps. Abnormally located ostia originate above the sinotubular junction, in the posterior aortic sinus, or eccentrically near a commissure.
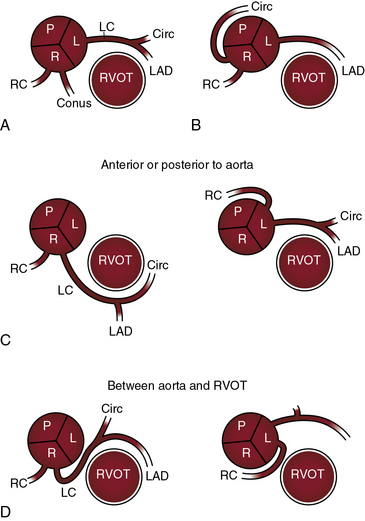
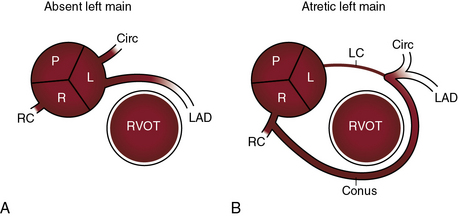
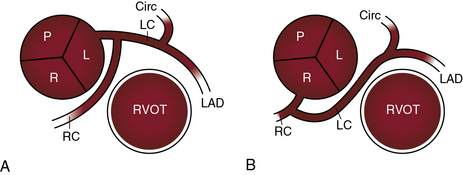
There are typically two coronary ostia, but three or four ostia are considered normal variants. A third ostium usually results from a separate conus branch in the right coronary sinus (see Figure 32-1A). Less commonly, three coronary ostia are the result of origin of the left anterior descending and circumflex coronary arteries from the left aortic sinus and of origin of the right coronary artery from the right aortic sinus, an arrangement that has been designated absent left main coronary artery (Figure 32-2A).
A relatively large left ventricular mass is served by both the left and the right coronary arteries. Either the right or the left coronary artery can be dominant, with dominance encompassing a wide range. Extreme dominance assumes that the dominant and nondominant coronary arteries arise from separate ostia in separate aortic sinuses, in contrast to dominance that results from a single coronary artery that originates from a single coronary ostium in a single aortic sinus (see subsequent).13
Congenital anomalies of the coronary arteries are the subject of comprehensive clinical and necropsy reviews.13–16 The widespread use of selective coronary angiography has gone far in establishing the types and prevalence of these anomalies (see Table 32-1).13,15,17 The incidence rate was 1.3% in 126,595 coronary arteriograms performed at the Cleveland Clinic.18 Transesophageal echocardiography19,20 and multislice computed tomographic coronary angiography21–25are established procedures for depicting the origin and course of anomalous coronary arteries.
Anomalous aortic origins of coronary arteries unassociated with congenital heart disease
Anomalous origins of coronary arteries that are unassociated with congenital heart disease are shown in Table 32-2.
Table 32-2 Congenital Anomalies of Coronary Arteries Unassociated with Congenital Heart Disease
| Anomalous Aortic Origin |
• Origin of left anterior descending and circumflex coronary arteries from separate ostia in the left aortic sinus (absence of left main coronary artery) • Origin of the left anterior descending coronary artery from the right aortic sinus or from the right coronary artery • Origin of the right coronary artery from the left aortic sinus, from posterior aortic sinus, or from left coronary artery |
| Anomalous Aortic Origin with Anomalous Proximal Course |
| Anomalous Origin of a Coronary Artery from the Pulmonary Trunk |
A normal coronary ostium is located in the middle of the aortic sinus and is considered anomalous when it is located above the sinotubular junction, in the posterior sinus, or in close proximity to an aortic commissure.4 In 30% to 50% of normal human hearts, a small conus artery arises from the right aortic sinus and is of no functional significance (Figure 32-1A).17,26 The circumflex coronary artery may arise from a separate ostium in the right aortic sinus and pass behind the aorta (Figure 32-1B) or may arise from the proximal right coronary artery and enter the left atrioventricular groove as if it were a proximal branch of the left coronary artery.18,27 Anomalous origin of the circumflex coronary artery is regarded as benign, but there is one report of myocardial ischemia in the absence of coronary atherosclerosis. Also regarded as benign is the absence of the circumflex coronary artery with a dominant right coronary artery that perfuses the lateral and posterolateral left ventricular walls.18
Origin of the left main coronary artery from the right aortic sinus is uncommon but clinically important.13,18,26 The right coronary artery and the left main coronary artery can arise from separate ostia in the right aortic sinus (see Figure 32-1C).28 The left main coronary artery passes either anterior or posterior to the right ventricular outflow tract (see Figure 32-1C) or between the right ventricular outflow tract and the aorta (see Figure 32-1D) or is within the ventricular septum (intramyocardial).13,18,26,29 The risk associated with passage of the left main coronary artery between the aorta and the right ventricular outflow tract has been convincingly established (see subsequent).
Absence of a left main coronary artery refers to a rare anomaly in which the left anterior descending and circumflex coronary arteries originate from separate ostia in the left aortic sinus (see Figure 32-2A).13,18,30 Both arteries are then normally distributed, so the arrangement is functionally benign.18
Atresia of the left main coronary artery includes ostial atresia, which is represented by an imperforate dimple, and atresia of the proximal course of the coronary artery, which is represented by a fibrous strand (see Figure 32-2B). A large conus branch originates from the right coronary artery and supplies the left anterior descending and circumflex coronary arteries (see Figure 32-2B).
Origin of the right coronary artery from the left aortic sinus (see Figures 32-1C and D, and 32-3B) represents about one quarter of ectopic coronary artery origins.13,28 Less common is origin of the right coronary artery from the posterior aortic sinus or from the left coronary artery.18 The functional significance of these arrangements does not depend on the anomalous origin but on whether the ectopic right coronary artery passes between the aorta and right ventricular outflow tract (see Figures 32-1D, and 32-3B and C).13,18,28
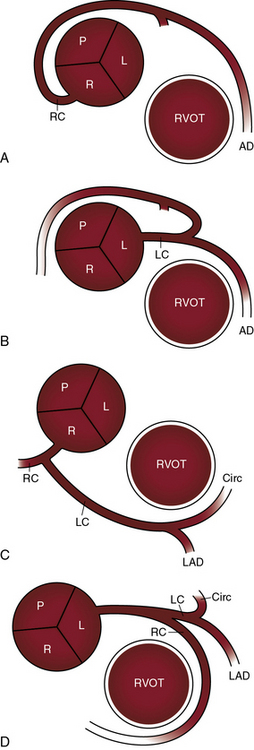
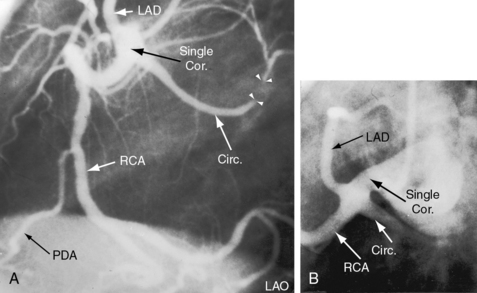
Single coronary artery has been known since 1903.31 The single coronary artery originates from a single ostium in the left or right aortic sinus and gives rise to the entire coronary circulation (Figures 32-4 and 32-5).13,18 A second coronary ostium is neither present nor implied. A single coronary artery unassociated with congenital heart disease divides into normally formed and normally distributed branches irrespective of the aortic sinus from which it originates (see Figure 32-4C and D).13,18 A single coronary artery associated with a congenital heart disease is characterized by branching patterns that bear no resemblance to normal.13 A single coronary artery functions normally unless a major branch is congenitally hypoplastic32 or passes between the aorta and right ventricular outflow tract (Figure 32-6).
Anomalous origin of a coronary artery from a systemic artery is relatively rare (see Table 32-2). The anomalous coronary artery can originate from the innominate, subclavian, internal mammary, or carotid or bronchial artery or from the descending aorta.
The Coronary Artery Surgery Study (1989) asked whether a coronary artery that originated anomalously from an aortic sinus was predisposed to atherosclerosis.33 The study concluded that atherosclerosis was more prevalent in anomalous circumflex coronary arteries than in matched controls with nonanomalous circumflex arteries.
The courses taken by anomalous coronary arteries and their branches are more important than their ectopic origins.26,29,34,35 These courses include: (1) passage anterior to the right ventricular outflow tract (see Figure 32-1C); (2) passage posterior to the aorta (see Figure 32-1C); (3) passage between the aorta and right ventricular outflow tract (see Figure 32-1D); and (4) intramyocardial passage (bridging).13,34–38 The clinical significance of bridging is uncertain.39 Angina pectoris, myocardial infarction, and sudden death are, with few exceptions, reserved for anomalous coronary arteries that pass between the aorta and the right ventricular outflow tract (see previous).13,26,29,34,40–42
Ischemic risk is determined by a number of variables in addition to the course of an anomalous coronary artery, namely13,26,28: (1) the coronary artery or branch that is involved; (2) the slit-like ostium and acute angulation of the origin of the ectopic coronary artery34,41,43; (3) coronary dominance28; and (4) the effect of strenuous exercise.26,34,43,44 Risk is greatest when the left main coronary artery arises from the right aortic sinus and courses between the aorta and right ventricular outflow tract (see Figure 32-1D)26,29,34,42,45 or when the left branch of a single coronary artery courses between the aorta and the right ventricular outflow tract (see Figure 32-6B). Risk is less when the right coronary artery originates from the left aortic sinus and passes between the aorta and the right ventricular outflow tract (see Figure 32-1D).28,41,43 Sudden death typically occurs during or immediately after physical exercise.26,29,34,42,44 Fatal impairment to coronary blood flow appears to be sporadic in light of the fact that highly trained competitive athletes perform intense physical exercise repeatedly and for many years before experiencing sudden death.46 It has been proposed that expansion of the aortic root and pulmonary trunk during exercise is responsible for an increase in acute angulation of the ectopic coronary artery and for flap-like closure of its slit-shaped ostium.13,26,46 An analogous risk may confront the fetus during the hemodynamic stress of labor and delivery. A stillborn term fetus that died during labor had at necropsy a right coronary artery that originated from the left aortic sinus.41 Exercise-induced dilation of the aortic root can compress an ectopic coronary artery against the base of the pulmonary trunk to which the infundibular septum is firmly anchored.13,26 This mechanism may account for sudden death when an ectopic coronary artery is not characterized by acute angulation or a slit-like ostium.28
Coronary arteries that originate normally from their respective aortic sinuses can have abnormal distal connections. A small coronary arterial fistula incidentally discovered during routine coronary arteriography is a case in point (see Chapter 22).18 Another example is a functionally benign distal intercoronary communication in which contiguous peripheral coronary artery branches form an open-ended circulation with bidirectional flow.18
Anomalous pulmonary arterial origins of coronary arteries13,18,47,48 are listed in Table 32-2. Anomalous origin of the left coronary artery from the pulmonary trunk is the most important (see Chapter 21).24 Anomalous origin of the right coronary artery from the pulmonary trunk is a rare anomaly that was first reported in 188549 and may be discovered incidentally at necropsy.50,51