27 Complications of Percutaneous Coronary Intervention
 Abrupt Closure
Abrupt Closure
Incidence
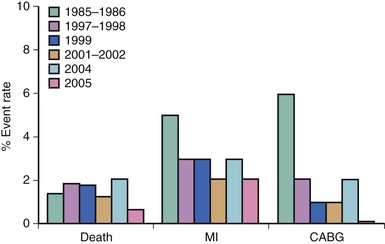
The incidence of abrupt closure (often called acute closure) during percutaneous coronary PCI has steadily decreased from 3% in the balloon angioplasty era to 0.3% in the current era (Fig. 27-1). The trend in decreasing acute closure rates corresponds to the increased utilization of stents and effective antithrombotics, including glycoprotein IIb/IIIa inhibitors, dual antiplatelet therapy, and direct thrombin inhibitors. The decrease in abrupt closure has resulted in a decrease in emergent coronary artery bypass grafting (CABG) and reduced periprocedural PCI mortality from 1.4% in 1985–1986 to 0.7% in 2006, most recently observed from the National Heart, Lung and Blood Institute (NHLBI) dynamic registry (Fig. 27-2).1
Mechanisms
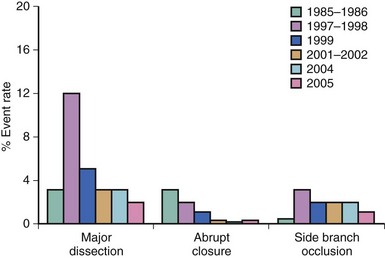
The most common mechanism of acute closure is dissection and injury to the media (Table 27-1, Fig. 27-3).2 Intramural hematomas can develop, along with intimal flaps, causing mechanical obstruction. With mechanical obstruction and the exposure of subintimal tissue, thrombus formation is often initiated. Vasoconstriction further complicates this milieu but is rarely a major mechanism of closure.3 While the predominate causes of acute closure in the pre-stent era were dissection (28%), thrombus (20%), or both (7%),4 the cause is indeterminate in almost 50% of patients.4,5 Patient factors predictive of abrupt closure include unstable angina, multivessel disease, and female gender.6,7 In examining the angiographic risk factors of dissection-mediated acute closure, proximal tortuosity was the strongest predictor, followed by American College of Cardiology (ACC) lesion grade C (Table 27-2), longer lesion length, and de novo stenosis. Similarly, risks of thrombotic closure include the presence of preexisting thrombus, degenerated vein grafts, and recent myocardial infarction.7 In the drug-eluting stent (DES) era, common causes of acute closure are stent-edge dissection and acute stent thrombosis. Stent-edge dissection occurs with oversizing of stents or aggressive balloon dilation at the edges, while acute stent thrombosis occurs either because of inadequate platelet inhibition or underexpansion of the stent.8 Recent analysis of the ACUITY trial found that independent predictors of early stent thrombosis included minimal luminal diameter, diabetes mellitus, and preprocedural administration of thienopyridines.9 Air embolism and the no-reflow phenomenon are also part of the differential diagnosis of abrupt closure (see sections for No Reflow and Air Embolism).
TABLE 27-1 Classification of Coronary Dissection
| Type | Description | Acute Closure (%) |
|---|---|---|
| A | Minor radiolucencies within lumen during angiography without dye persistence | 0–2 |
| B | Parallel tracks or double lumen separated by radiolucent area during angiography without dye persistence | 2–4 |
| C | Extraluminal cap with dye persistence | 10 |
| D | Spiral luminal filling defects | 30 |
| E | New persistent filling defects | 9 |
| F | Non-E types leading to impaired flow or total occlusion | 69 |
Reproduced with permission from Klein LW. Catheter Cardiovasc Intervent. 2005;64:395–401.
TABLE 27-2 ACC/AHA Lesion Classification System
| Type A |
| Type B |
| Type C |
B1, one adverse characteristic; B2, ≥2 adverse characteristics.
Prognostic Significance
Most data regarding outcomes of patients experiencing abrupt closure are derived from the pre-stent era. In studies from the balloon angioplasty era, 6% of patients died, 36% suffered nonfatal myocardial infarction, and 30% were referred for emergency CABG.4
Treatment
The first priorities are to stabilize hemodynamics and relieve ischemia. Vasopressors, ionotropes and possibly intra-aortic balloon pump (IABP) insertion should be considered for hemodynamic instability. Extreme vagal reactions may also cause bradycardia or hypotension and should be treated with atropine, intravenous fluid boluses, and vasopressors if necessary. Immediate recognition and treatment of electrical instability with antiarrhythmic medications and cardioversion is imperative.3 Expeditious balloon inflation to reestablish antegrade flow must be quickly attempted. Urgent stenting is usually required to stabilize the dissection. Glycoprotein IIb/IIIa antagonists can be helpful if thrombus is responsible for the acute closure. Prospective use of abciximab in elective or urgent PCI was compared in both balloon angioplasty and stenting cohorts in the EPISTENT trial, showing that abciximab decreases rates of abrupt closure and side-branch loss in both cohorts.10 The utility of glycoprotein IIb/IIIa inhibitors as a bailout is controversial, as the data support its use upstream to PCI.10,11 In the case of persistent acute closure, one should consider the use of intravascular ultrasound (IVUS) to more clearly define the pathology. IVUS can document the presence and extent of dissection. Multiple stents may be required to restore patency and achieve an adequate result. If the IVUS findings are suspicious for thrombus, aspiration thrombectomy may be valuable and the addition of a glycoprotein IIb/IIIa inhibitor should be considered. Intracoronary thrombolytic delivery has been used as a bailout for abrupt closure, but it is associated with significant morbidity and is not recommended.6
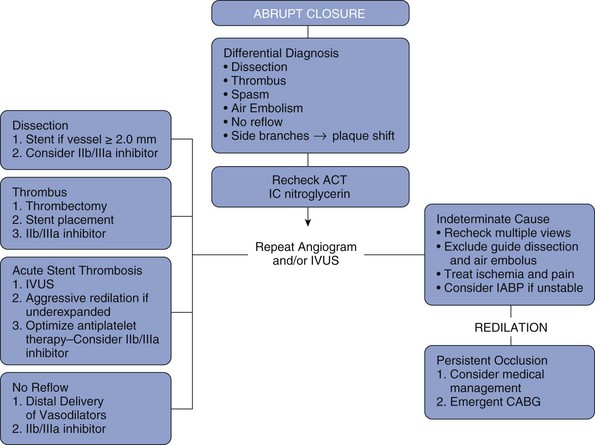
Factors affecting the inflow and outflow should be investigated. The use of multiple angiographic views, IVUS, and selective distal injections may better delineate the anatomy. IVUS is particularly helpful in confirming suspected stent-edge dissections or stent underexpansion. Guide-catheter dissections may cause poor inflow and can easily be overlooked. Pressure dampening, ventricularization, ECG changes, or severe ischemic pain may point to ostial guide catheter dissection. Distal wire dissections that occlude outflow may require stenting even if the distal vessel is of small caliber. In the pre-stent, balloon angioplasty era, approximately 40% of cases with acute closure ultimately achieved procedural success.6 The predominate treatment was repeat balloon dilation with prolonged inflation facilitated by autoperfusion balloon catheters. Historically, the use of stents as bailout devices for abrupt or impending closure dramatically decreased the need for emergent CABG.12 Although no randomized trial has been performed, the dramatic decrease in urgent and emergent CABG, from 3% to 0.7% in the NHLBI dynamic registry, was clearly associated with the use of stents and more effective anticoagulation, primarily glycoprotein IIb/IIIa inhibitors.1 Clearly in the case of edge dissections, additional stents are required to cover the dissection and maintain vessel patency. Acutely thrombosed stents due to underexpansion require immediate dilation to achieve stent apposition and repeat IVUS to verify optimal stent deployment. Patients with a successful outcome following treatment of abrupt closure require close monitoring in an intensive care setting. If needed, intra-aortic balloon counterpulsation should be initiated until the patient is stable. Serial cardiac enzyme assessments, ECGs, and echocardiography to assess the extent of myocardial damage are also recommended (see Fig. 27-4 for an algorithm outlining the management of acute vessel closure).
 Coronary Perforation
Coronary Perforation
Incidence
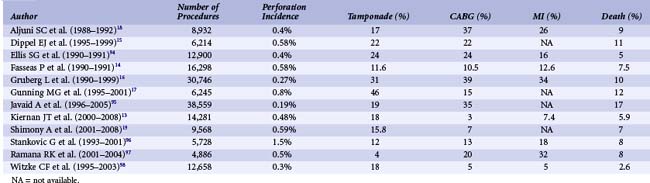
Coronary perforation is a serious complication with an incidence ranging from 0.19% to 3% of cases (Table 27-3). Lesions associated with perforation are more complex in nature. ACC type B or C, calcified lesions, or chronic total occlusions are more likely to sustain perforation.13,14 Women and the elderly are at greater risk of perforation.4
Mechanism
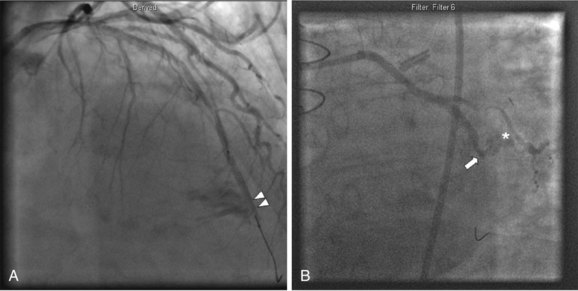
There are two basic mechanisms of perforation: guide wire penetration and vessel rupture. Vessel rupture is usually caused by balloon or stent mismatch with oversizing of the dilatation catheter. Occasionally, an appropriate sized catheter will result in perforation, due to extensive dissection, lack of vessel wall integrity, or calcification. Early balloon angioplasty studies found that when the balloon : artery ratio became greater than 1.2 : 1, the risk of perforation increased.4 Use of atherectomy devices such as eximer laser or rotational atherectomy also increases the risk of perforation.15 Recanalization of chronic total occlusions has become a common setting for perforation, usually owing to small guidewire perforations, particularly with the increasing use of stiffer and hydrophilic guidewires.13 Ellis-graded perforations (Table 27-4) fall into three classes of severity ranging from small endovascular leaks into the adventia (grade I) to frank extravasation into the pericardial space (grade III). Grade I perforations are frequently caused by guidewires, but atheroablative devices as well as stents can cause small endovascular leaks. Occasionally guidewires can cause very large distal perforations; therefore extreme vigilance should be maintained, particularly in using stiff and hydrophilic guidewires in distal tortuous vessels. Grade II and III perforations are usually caused by high-pressure balloon inflations, oversized balloon catheters or stents, or the use of atheroablative devices (Fig. 27-5).
TABLE 27-4 Ellis Classification of Coronary Perforations
| Classification | Description | Possible Clinical Sequelae |
|---|---|---|
| I | Focal extraluminal crater without extravasation; limited to media or adventitia | Usually benign, may rarely cause delayed cardiac tamponade |
| II | Pericardial or myocardial blush without contrast in pericardium; limited extravasation producing patch of blushing or staining within the myocardium or pericardium | |
| III | Persistent extravasation with streaming or jet of contrast | High risk, increased morbidity and mortality |
| IIIA | Directed toward pericardium | High risk of acute cardiac tamponade |
| IIIB | Directed toward myocardium (e.g., ventricular cavity) | More benign course; possible fistula formation |
Reproduced with permission from Klein LW. Catheter Cardiovasc Intervent. 2006;68:713–717.
Prognosis
Perforation severity correlates with worsening prognosis. Grade III perforations can quickly result in cardiac tamponade, rapid hemodynamic collapse, myocardial infarction (MI), and/or death4,13–19 (Table 27-3). Referral for emergency CABG is often indicated.
Diagnosis
Given the dire consequences of coronary perforation, the interventionist must be especially attuned to recognizing this complication early. Patients may experience severe chest pain, dizziness, or nausea disproportional to symptoms typically associated with balloon inflation. There may be persistent ST-segment changes after balloon inflation. Vasovagal reactions may also accompany perforations, along with severe bradycardia and hypotension.20 Awareness and vigilance post-PCI is vital, since cardiac tamponade as late as 24 hours post-PCI has been reported.4,21 Late tamponade risk can be minimized with careful “final” angiograms visualizing all instrumented vessels and their branches.
Management
Grade I perforations can generally be treated with reversal of anticoagulation and/or prolonged balloon inflation at or proximal to the perforated vessel segment. Guidewire perforations are often best treated by balloon occlusion but can also be treated with the delivery of occlusive coils, fat, or beads. Occasionally, grade I perforations can resolve without an intervention, but small endovascular leaks may persist and require the use of a covered stent or referral to emergent CABG. Grade III perforations are catastrophic and more likely require urgent pericardiocentesis, deployment of a polytetraflouroethylene (PTFE)-covered stent, and/or referral for emergent surgery.20
Once a perforation occurs, the first step is to remain calm and advance a balloon from the guide catheter across the perforation without losing guidewire position. If the perforation has resulted from a guidewire, balloon inflation proximal to the perforation is indicated. This highlights an essential fundamental rule of interventional cardiology: a balloon should remain in the guide or within the lesion following any inflation until angiography confirms that there is no perforation. When perforation is recognized, balloon expansion to a pressure sufficient to occlude flow (usually 2–4 atm) is the first and most urgent step. Once the vessel is occluded, the patient`s hemodynamics may normalize; however, aggressive treatment with intravenous fluids, atropine, vasopressors, and perhaps an intra-aortic balloon pump may be required. In treating perforations, anticoagulation is immediately discontinued. If heparin was used as the anticoagulant, reversal with protamine is usually indicated. Glycoprotein IIb/IIIa inhibitors must also be stopped and abciximab should be reversed with an infusion of platelets. The effects of tirofiban and eptifibatide cannot be reversed with platelet infusions, but these agents have a shorter half-life. Although IIb/IIIa inhibitors can increase bleeding complications, they do not increase the incidence or severity of perforations.15 Direct thrombin inhibitors such as bivalirudin are increasingly utilized for peri-PCI anticoagulation, and the short half-life of this anticoagulant is advantageous in sealing wire perforations. Infusion of fresh frozen plasma is the only means of reversing anticoagulation with bivalirudin; this, however, requires a time delay to thaw the blood products.22
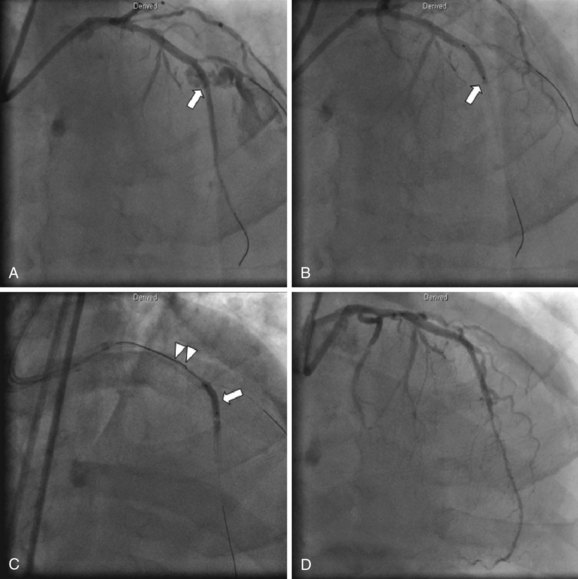
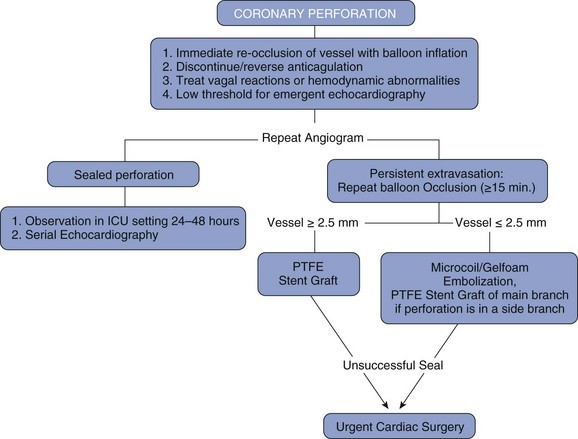
The presence of coronary perforation should also trigger urgent echocardiography. If a large pericardial effusion is present associated with cardiac tamponade physiology, emergent pericardiocentesis is indicated. Should echocardiography not be available, the diagnosis of tamponade on clinical grounds, use of right heart catheterization, or fluoroscopy of the heart’s borders may be helpful. A major advance in the treatment of coronary perforation is the availability of PTFE membrane-covered stents. Prior to the advent of PTFE stents, the presence of a grade III perforation often required emergency CABG, which carried significant mortality.21 The currently available PTFE stent is a distensible microporous PTFE membrane layered between two bare metal stents. Deployment of the stent graft excludes the perforation, possibly at the cost of occluding side branches. The initial experience using PTFE grafts was reported in a small case series demonstrating a decrease in cardiac tamponade and emergency CABG.23 PTFE-covered stents are bulky and delivery into tortuous vessels can be challenging. A separate guide catheter for delivery of the PTFE-covered stent may be needed, as most guide catheters cannot accommodate both an angioplasty balloon and PTFE-stent graft (Fig. 27-6). Therefore a two-guide technique has been developed wherein contralateral access is established and a separate guide catheter is used to deliver the stent. A wire from the second guide catheter is advanced down the coronary vessel and the angioplasty balloon is momentarily deflated to allow guidewire passage. The PTFE stent is then quickly advanced across the perforation and deployed after the removal of the angioplasty balloon. This technique employing two guide catheters has been documented to decrease the rate of adverse events.24 Side-branch vessels near the perforation site may be excluded by the PTFE-covered stent, which may result in a periprocedural MI.23 IVUS should be used routinely to verify adequate expansion of the covered stent, since deploying this dual stent layer may require aggressive (≥18 atm) but judicious postdilation. Occasionally, collateral filling may cause persistent extravasation despite exclusion of the perforation with a stent graft and surgical management, or occlusion of the supplying collateral may be required. Perforations in small vessels can be addressed with either additional prolonged balloon inflations or the injection of thrombin, polyvinyl alcohol, Gelfoam, collagen, or the embolization of microcoils or beads.25,26 (See Fig. 27-7 for a suggested strategy for the management of coronary perforation.)
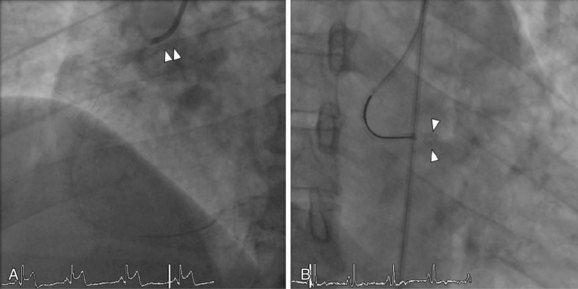
 Device Embolization
Device Embolization
Incidence
Embolization of equipment such as coronary stents and guidewire fragments is a potentially catastrophic complication of PCI. Stents are the most common devices embolized, with an incidence ranging from 3% for first-generation hand-crimped devices to a much lower 0.32% for current stent delivery systems.27,28
Mechanism
Extreme tortuosity and calcification increase the risk of stent embolization due to dislodgement from delivery balloons. For this reason, stents are more frequently lost in the right coronary and circumflex arteries and less commonly in the left anterior descending artery.27,29 In the initial experience using Palmaz-Shatz stents, manually crimped stents were found to have a relatively high embolization rate of 3%.28
Treatment
There are numerous approaches to the removal of embolized stents. If possible, maintain guidewire position through the center of the stent. This will facilitate retrieval using one of many commercially available snares (Fig. 27-8). Another option is to advance a small-diameter balloon through the unexpanded stent and attempt to drag it back into the guide catheter. A third approach is to pass a second wire alongside the embolized stent, attempting to enter one of the struts, and then to attach a single torquing device to both wires used in order to twist the wires together. Then the wire-wrapped stent is withdrawn from the artery. A fourth approach is to deploy the embolized stent in its unintended location. Retrieval devices such as biliary forceps or bioptomes can easily damage the arterial wall and should be avoided or handled with great care. Deploying a new stent alongside the embolized stent such that the dislodged stent is embedded into the arterial wall is a reasonable option should retrieval be difficult, but this technique may be associated with an elevated risk of periprocedural MI, death, and referral to CABG.30