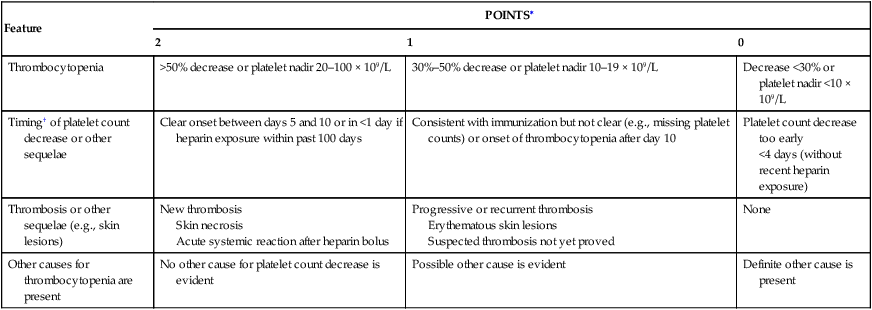
Janice N. Thai, Magdiel Trinidad-Hernandez and Joseph L. Mills A clinical scoring system (4 Ts) proposed by Warkentin and Heddle in 2003 can be used to assess the pretest probability of HIT type II based on four characteristic features: the degree of thrombocytopenia, the timing of the onset, the presence of new or progressive thrombosis, and whether an alternative cause of thrombocytopenia is likely (Table 1). A moderate to high pretest probability mandates cessation of heparin and administering an alternative anticoagulant. With a low pretest probability, other reasons for thrombocytopenia or thrombosis should be sought. TABLE 1 Estimating the Pretest Probability of Heparin-Induced Thrombocytopenia: The Four Ts ∗Maximum possible score is 8. Pretest probability score: 6–8 = high; 4–5 = intermediate; 0–3 = low. †First day of immunizing heparin exposure is considered day 0; the day the platelet count begins to fall is considered the day of onset of thrombocytopenia. It generally takes 1–3 more days until an arbitrary threshold defining thrombocytopenia is passed. From Warkentin TE, Heddle NM: Laboratory diagnosis of immune heparin-induced thrombocytopenia, Curr Hematol Rep 2:148–157, 2003.
Complications of Heparin Anticoagulation Therapy
Nonbleeding Complications
Heparin-Induced Thrombocytopenia
Feature
POINTS∗
2
1
0
Thrombocytopenia
>50% decrease or platelet nadir 20–100 × 109/L
30%–50% decrease or platelet nadir 10–19 × 109/L
Decrease <30% or platelet nadir <10 × 109/L
Timing† of platelet count decrease or other sequelae
Clear onset between days 5 and 10 or in <1 day if heparin exposure within past 100 days
Consistent with immunization but not clear (e.g., missing platelet counts) or onset of thrombocytopenia after day 10
Platelet count decrease too early
<4 days (without recent heparin exposure)
Thrombosis or other sequelae (e.g., skin lesions)
New thrombosis
Skin necrosis
Acute systemic reaction after heparin bolus
Progressive or recurrent thrombosis
Erythematous skin lesions
Suspected thrombosis not yet proved
None
Other causes for thrombocytopenia are present
No other cause for platelet count decrease is evident
Possible other cause is evident
Definite other cause is present
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
