Chapter 27 Complete Transposition of the Great Arteries
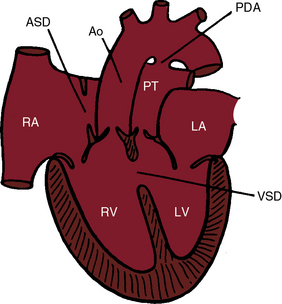
In 1797, Matthew Baillie called attention to “a singular malformation in which the pulmonary artery arises from the left ventricle and the aorta from the right ventricle.”1 Seventeen years later, John Farre2 used the term transposition to characterize Baillie’s singular malformation. Each great artery is placed across the ventricular septum: positio means placed, trans means across. The term transposition of the great arteries is used herein in its original sense to signify that the aorta arises from the morphologic right ventricle and the pulmonary trunk arises from the morphologic left ventricle—ventriculoarterial discordance (Figures 27-1 through 27-4).3–6 The designation “complete” means that ventriculoarterial discordance is associated with atrioventricular concordance. The single discordance between ventricles and great arteries makes the transposition physiologically complete. These alignments result in a unique extrauterine circulation in which two independent circulations function in parallel (Figure 27-5). Congenitally corrected transposition (see Chapter 6) refers to atrioventricular and ventriculoarterial discordance. The double discordance physiologically corrects the transposition, which is therefore not complete. Accordingly, there is one circulation in series, as in the normal heart. The term malposition of the great arteries refers to abnormal spatial relations but concordant ventricular alignments.7,8
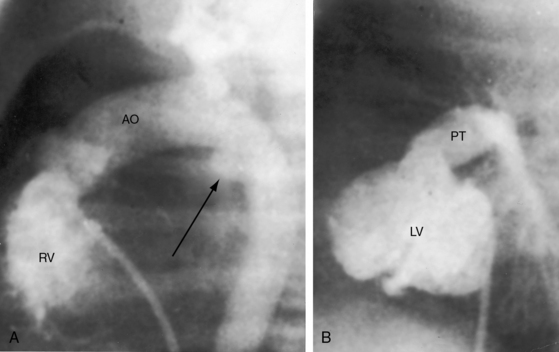
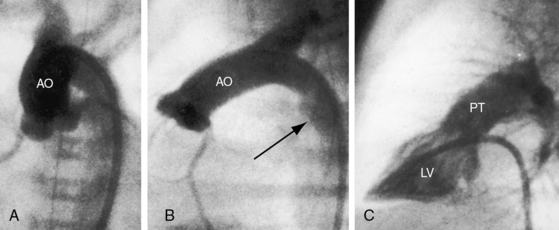
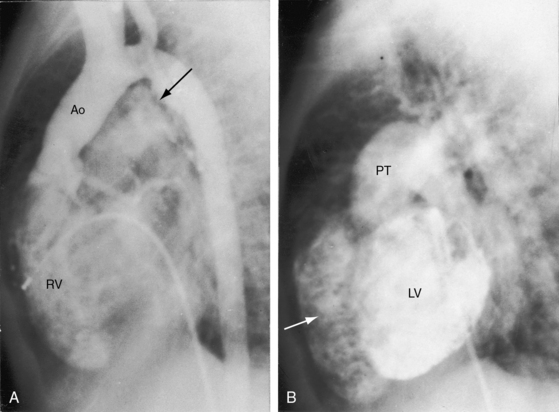
d-Transposition and complete transposition are synonymous. Because d- refers to a dextro (rightward) bend in the bulboventricular loop, the designation is appropriate because virtually all examples of complete transposition of the great arteries are accompanied by a d-ventricular loop (see Figures 27-2A, and 27-4A). Segmental analysis identifies situs solitus (S), a dextro-ventricular loop (d), and a rightward (d) and anterior aorta (see Figures 27-2A, 27-3, and 27-4). In about one third of cases, the aorta is anterior and to the left, directly anterior, or rarely, posterior to the pulmonary trunk.3,5 The great arteries rise in parallel and do not cross, as in the normal heart (see Figures 27-1, 27-34, and 27-35).9 Anomalous origin of the left subclavian artery from the pulmonary artery has been reported in association with a right aortic arch.10
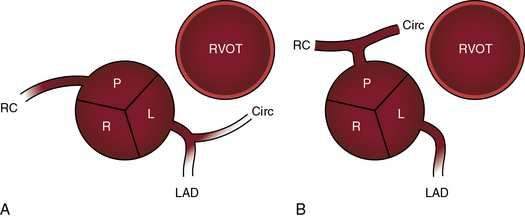
The origin and course of the coronary arteries are physiologically unimportant but anatomically pivotal because of the arterial switch operation (see Chapter 32).11–14 In complete transposition with situs solitus, the morphologic right coronary artery is concordant with the morphologic right ventricle and the morphologic left coronary artery is concordant with the morphologic left ventricle.15 Two aortic sinuses face the right ventricular outflow tract (Figure 27-6), irrespective of the spatial relations between the ascending aorta and the pulmonary artery.13,14 The two facing sinuses are the left sinus and the posterior sinus.13,14 The right aortic sinus does not face the right ventricular outflow tract (see Figure 27-6). Rarely, both coronary arteries are intramural.16 In complete transposition with situs inversus, the anatomy of the coronary arteries has not been well established.17
Dual sinus origin indicates that a coronary artery arises from each of the two facing sinuses (see Figure 27-6) and accounts for about 90% of cases; in most of these cases, the left main coronary artery arises from the left aortic sinus and gives rise to the anterior descending and circumflex coronary arteries, and the right coronary artery arises by a single ostium from the posterior aortic sinus (see Figure 27-6A). In the less common type of dual sinus origin, the left anterior descending coronary artery originates from the left aortic sinus, and the circumflex and right coronary arteries originate by a single ostium in the posterior aortic sinus (see Figure 27-6B.) Single sinus origin indicates that both coronary arteries arise from one of the two, but not both, facing sinuses by a single ostium or by multiple ostia. In a small minority of cases of single sinus origin, the left anterior descending, circumflex, and right coronary arteries arise by ostia in the posterior aortic sinus. The sinus node artery originates from the proximal right coronary artery and courses upward and to the right, partially embedded in the limbus of the atrial septum. An aberrant anterior descending coronary artery may pass intramurally between the aortic root and pulmonary trunk.18
The incidence rate of complete transposition of the great arteries is estimated at 1 in 2300 to 1 in 5100 live births.19 The malformation represents approximately 5% to 8% of congenital cardiac malformations but accounts for 25% of deaths from congenital heart disease in the first year of life. The morphogenesis of the ventriculoarterial discordance that characterizes complete transposition focuses on the conus as the crucial connection between the ventricles and the great arteries.20 The segmental components of the heart, the atria, the ventricles, and the great arteries, appear at different developmental stages.3 The straight heart tube is formed from primordia of the trabeculated portions of both ventricles. During looping, the atria are at the caudal end of the embryonic tube, and the conus is at the cephalic end of the tube. When the truncus appears, the developing heart has three segments: atrial, ventricular, and arterial.3 The ventral end of the arterial segment is continuous with the conus, and the dorsal end is continuous with the aortic arches. The spiral division that develops within the truncus progresses from the aortic arches toward the truncal ridges. Conal development then becomes pivotal.20 Rarely, the aortic arch is double.21 The subaortic portion of the conus persists in complete transposition of the great arteries, and the subpulmonary conus is absorbed (conal inversion). The aortic valve moves anteriorly, and the pulmonary valve moves inferoposteriorly into fibrous continuity with the mitral valve. The maldeveloped conus is inappropriate for the ventricular loop, so the great arteries are discordant relative to their ventricles of origin. Pulmonary/mitral continuity exists because a left-sided subpulmonary conus is absent, and aortic/tricuspid discontinuity exists because a right-sided subaortic conus is present. Aortic/tricuspid discontinuity causes the aortic valve to lie superior to the pulmonary valve and causes the ascending aorta to lie parallel to the pulmonary trunk.
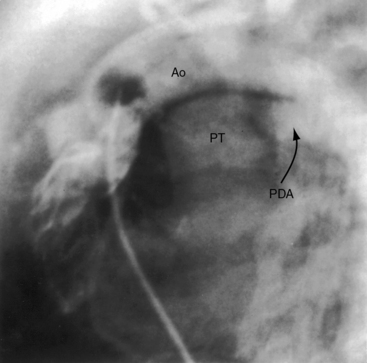
The ductus arteriosus is usually closed or insignificantly patent in complete transposition (Figures 27-3, 27-4, and 27-7)22 but is occasionally widely open (Figure 27-8).23 Interatrial communications take the form of a patent foramen ovale or an ostium secundum atrial septal defect.5,6,24 Rarely, the foramen ovale closes prematurely.25 Ventricular septal defects are inlet, muscular, perimembranous, or infundibular.26 Inlet defects caused by malalignment with the atrial septum result in straddling of the tricuspid valve. Perimembranous ventricular septal defects extend into the inlet septum and into the muscular septum. Infundibular septal defects result from malalignment of the infundibular septum, which is shifted leftward and posterior or rightward and anterior, and the malaligned defect can be subaortic, subpulmonary, or doubly committed.26
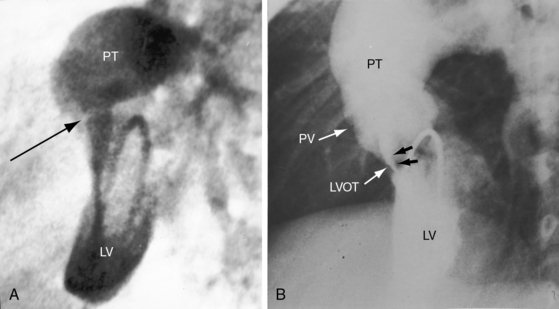
Pulmonary stenosis is represented by obstruction to left ventricular outflow and occurs in approximately 15% of cases of complete transposition.5,27 Fixed subpulmonary stenosis is characterized by a circumferential fibrous membrane or diaphragm (Figure 27-9A), a fibromuscular ridge, herniation of tricuspid leaflet tissue, anomalous septal attachments of the mitral valve, accessory mitral leaflet tissue, tissue tags from the membranous septum, or hypertrophy of the anterolateral muscle bundle.5,27 Leftward and posterior deviation of the infundibular septum causes tunnel or tubular subpulmonary stenosis.26 Pulmonary valve stenosis is uncommon.27
Dynamic subpulmonary stenosis develops during the first few weeks of life and is associated with systolic anterior motion of the anterior mitral leaflet (see Figures 27-9B, and 27-36).28 The obstruction is dynamic because the degree varies spontaneously and is augmented by isoproterenol and reduced by beta blockade.28 The development of dynamic subpulmonary stenosis coincides with a fall in pulmonary vascular resistance and a fall in left ventricular systolic pressure in the face of persistent systemic systolic pressure in the adjacent right ventricle. Systemic right ventricular pressure results in systolic movement of the base of the ventricular septum into the outflow tract of the adjacent low-pressure left ventricle. Systolic anterior motion of the anterior mitral leaflet is then caused by the Venturi effect that is generated by hyperkinetic ejection of a volume-overloaded left ventricle into a low-resistance pulmonary vascular bed (see Figure 27-9B).
Subaortic stenosis is the result of rightward and anterior deviation of a malaligned infundibular septum.26,29,30 Aortic arch anomalies are represented by hypoplasia, coarctation, and interruption and have been attributed to reduced flow during morphogenesis.31
Juxtaposition of the atrial appendages refers to an anomaly in which both atrial appendages or the left appendage and part of the right appendage are adjacent to each other (juxtaposed) on the same side of the heart. Juxtaposition is a rare anomaly that is strongly associated with complete transposition of the great arteries32–35 and occurs in 2% to 6% of cases of anatomically corrected malpositions.32 Juxtaposition reduces the size and volume of the right atrium. Left-sided juxtaposition is six times as frequent as right-sided juxtaposition. There is female preponderance with juxtaposition of the atrial appendages, in contrast to marked male preponderance in complete transposition of the great arteries without juxtaposition (see section History).34
Tricuspid valve abnormalities occur in about 30% of patients with complete transposition and inlet ventricular septal defects.36 These abnormalities include straddling or overriding of chordae, overriding of the tricuspid annulus, abnormal chordal attachments, and less commonly, tricuspid valve dysplasia, accessory tricuspid tissue, and double orifice tricuspid valve.36 Mitral valve abnormalities occur in 20% to 30% of necropsy specimens of complete transposition and include a cleft anterior leaflet, straddling of the mitral valve, abnormal size or position, anomalous mitral tissue strands, redundant mitral valve tissue, and abnormal papillary muscles.37,38
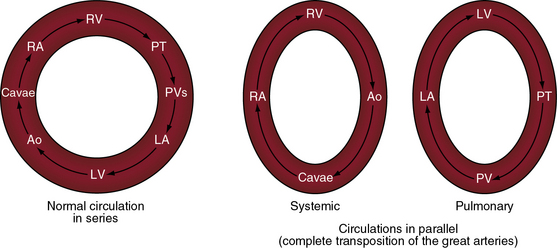
Matthew Baillie1 described the anatomic features of a singular malformation. The malformation is even more singular in physiologic terms. The normal heart is associated with a single circulation in series (see previous and Figure 27-5), with sequential flow from the right ventricle into the pulmonary artery, pulmonary veins, left atrium, left ventricle, aorta, systemic venous bed, venae cavae, and right atrium and back to the right ventricle. Blood at any given location must traverse both sides of the circulation before returning to that location (see Figure 27-5). In complete transposition of the great arteries, two circulations are in parallel (see Figure 27-5). The systemic circulation is characterized by flow from the right ventricle into the aorta, systemic venous bed, venae cavae, and right atrium and back to the right ventricle. The pulmonary circulation is characterized by flow from the left ventricle into the pulmonary artery, pulmonary capillary bed, pulmonary veins, and left atrium and back to the left ventricle. Blood within the systemic circulation recirculates within the systemic circulation, and blood within the pulmonary circulation recirculates within the pulmonary circulation. The two circulations do not cross unless they are joined by communications at the atrial, ventricular, or great arterial level, on which survival depends. These communications permit blood from the systemic circulation to enter the pulmonary circulation for oxygenation and permit oxygenated blood from the pulmonary circulation to enter the systemic circulation.
The net volume of blood exchanged between the systemic and pulmonary circulations must be isovolumetric over short periods of time or the donor circulation is rapidly depleted and the recipient circulation is rapidly overloaded. The amount of blood exchanged between the two parallel circulations is small relative to the volume that recirculates within each circulation. The volume of effective bidirectional mixing depends on the location and size of the communication that joins the two circulations and on the magnitude of pulmonary blood flow.39 Survival is tightly coupled to the delicate interplay between the intercirculatory communications and the pulmonary blood flow. In a neonate with complete transposition, a restrictive foramen ovale, and a nonpatent ductus arteriosus, the circulations are in parallel, as illustrated in Figure 27-5. The tendency for neonatal pulmonary vascular resistance to fall prompts an increase in pulmonary blood flow, but that advantage is lost because the oxygenated blood cannot enter the systemic vascular bed. The value of an adequate interatrial communication is dramatized by the immediate response to balloon atrial septostomy.40,41 An adequate interatrial communication permits quantitatively equal bidirectional shunting that is right-to-left during ventricular diastole and left-to-right during ventricular systole.39,42,43
An adequately sized ventricular septal defect permits bidirectional shunting that is determined by instantaneous pressure differences between the two ventricles. When pulmonary vascular resistance is low, there is preferential right-to-left systolic shunting into the low resistance pulmonary circulation and preferential left-to-right diastolic shunting away from the volume-loaded left ventricle. A large patent ductus arteriosus (see Figure 27-8) is initially accompanied by bidirectional flow that is replaced by virtually exclusive systemic-to-pulmonary flow as pulmonary vascular resistance falls.23 The temporary value of ductal patency is witnessed by the response to prostaglandin-induced ductal dilation as a pharmacologic bridge to balloon septostomy.44 Because ductal flow is essentially unidirectional, the pulmonary circulation becomes volume overloaded with no egress except a through restrictive foramen ovale.
Low pulmonary vascular resistance with an increase in pulmonary blood flow provides a large volume of oxygenated blood for intercirculatory mixing.39 Elevated pulmonary vascular resistance and pulmonary stenosis decrease pulmonary blood flow and result in a smaller volume of oxygenated blood for intercirculatory mixing. The increase in left ventricular afterload incurred by pulmonary vascular disease or pulmonary stenosis results in a reduction in left ventricular compliance that adversely affects intracardiac mixing.39,42 Hypoxemia provokes a fall in systemic vascular resistance and an increase in the volume of unsaturated blood recirculating in the systemic vascular bed.39,42
The geometry of the left ventricle in complete transposition is governed by the volume overload imposed by increased pulmonary blood flow and the pressure overload imposed by increased pulmonary vascular resistance or pulmonary stenosis. Right ventricular free wall thickness exceeds normal in the neonate and outstrips left ventricular wall thickness. Septal thickness and right ventricular free wall thickness then increase in parallel, so the septum becomes disproportionately thick relative to the left ventricular wall.45–49 When the ventricular septum bows into a low-pressure left ventricle, the right ventricle becomes spherical and the left ventricle resembles a prolate ellipsoid.50
When left ventricular pressure is elevated, the septum flattens or bows into the right ventricle, resulting in a more normal septal position and better left ventricular function.45 During the first few weeks of life, left ventricular function is normal, but mass does not increase in proportion to volume,51 a discrepancy that is responsible for decreased left ventricular function. The right ventricle performs normally at birth, but its contractility and ejection fraction then decline.51,52 A morphologic subaortic right ventricle is ill-equipped to support the systemic circulation because of the adverse effects of its mass, geometry, and the coronary circulation (see Chapter 6).
Pulmonary vascular disease is prevalent in patients with complete transposition of the great arteries, especially in the presence of a nonrestrictive ventricular septal defect53–55 or a large patent ductus arteriosus.23 Grade 3 or 4 Heath-Edwards changes of pulmonary vascular disease are found in 20% of infants before 2 months of age, and in about 80% after 1 year.56 A reduction in number of intra-acinar pulmonary arteries has been identified with quantitative morphomeric studies.57,58 Vasoconstriction of pulmonary arterioles induced by hypoxemic blood carried in the systemic arterial collaterals accelerates the pulmonary vascular disease.59 Early pulmonary vascular disease is more prevalent with a nonrestrictive ventricular septal defect and complete transposition than with an equivalent isolated ventricular septal defect.56,59,60 Even with an isolated atrial septal defect, the incidence rate of pulmonary vascular disease is about 6%, although progression is slower.53,61
History
Males outnumber females with a ratio as high as 4:1,5,62 unless there is juxtaposition of the atrial appendages.34 Complete transposition of the great arteries seldom occurs in firstborns; but in offspring of mothers who have had three or more pregnancies, a twofold increase in incidence rate has been reported. Familial recurrence of concordant cardiac defects within affected family members supports monogenic or oligogenic inheritance in selected pedigrees.63 Complete transposition and congenitally corrected transposition sometimes segregate in the same family, probably because of monogenic transmission, which supports a pathogenetic link between complete transposition and disorders of looping.63
Cyanosis begins as early as the first day of life in more than 90% of infants with an intact ventricular septum.19 Severe pulmonary stenosis or atresia results in intense neonatal cyanosis. Mild cyanosis with delayed onset is a feature of complete transposition with a nonrestrictive ventricular septal defect64 or a patent ductus arteriosus.23 A large isolated ductus (see Figure 27-8) is associated with severe congestive heart failure in the first few days of life, and spontaneous closure results in a sudden fall in systemic arterial oxygen saturation, rapid clinical deterioration, and death.23 Dynamic subpulmonary stenosis or a rise in pulmonary vascular resistance curtails pulmonary blood flow and alleviates congestive heart failure, but at the expense of increasing hypoxemia. Pulmonary stenosis is occasionally responsible for hypercyanotic spells characterized by intense cyanosis, tachypnea, extreme irritability, and hypothermia but seldom by loss of consciousness.28 Squatting is rare. Neonatal survival is tightly coupled to the delicate interplay between the intercirculatory communications and pulmonary blood flow.65 An older infant depends for survival on adequate pulmonary blood flow. Outcome is bleak, with an overall death rate of 30% in the first week, 50% in the first month, and 90% in the first year.24,66,67
Survival is poorest when the foramen ovale is restrictive, the ventricular septum is intact, and the ductus is closed. The salutary effect of an adequately sized interatrial communication is underscored by the immediate response to balloon septostomy (see previous),41 after which 75% of patients survive for 6 months, 65% survive for 1 year, and many survive into their teens.40 A nonrestrictive ventricular septal defect with pulmonary vascular disease carries a 6-month survival rate of about 30% and a 1-year survival rate of about 20%. Moderate pulmonary stenosis improves longevity by regulating pulmonary blood flow, with three quarters of patients surviving for a year or more. Most of those who reach their teens have a nonrestrictive ventricular septal defect with pulmonary vascular disease or pulmonary stenosis. Sporadic examples of unusual longevity have been recorded in the third, fourth, and fifth decades (see Figure 27-7),31,68,69 and complete transposition was confirmed at necropsy in a 56-year-old patient.70 Brain abscess is rare in patients younger than 2 years of age but is believed to have a predilection for complete transposition of the great arteries and Fallot’s tetralogy.71