Chapter 163
Compartment Syndrome
Jayer Chung, J. Gregory Modrall
Compartment syndrome is a surgical emergency and a recognized complication of several conditions treated by vascular surgeons. Failure to arrive at a timely diagnosis increases the risk for short- and long-term morbidity, including limb loss or permanent disability. Conversely, appropriate recognition and management of a compartment syndrome will optimize the chances of a full recovery. This chapter will address the pathogenesis, diagnosis, and treatment of compartment syndrome of the lower leg and other less common anatomic sites. Abdominal compartment syndrome is discussed in Chapter 158.
Pathogenesis of Compartment Syndrome
Local Hemodynamics of Compartment Syndrome
The unifying feature of all compartment syndromes, regardless of etiology or anatomic location, is an increase in intracompartmental pressure (ICP) within an unyielding fascial envelope that impairs tissue perfusion.1 The adverse consequence of elevated ICP on tissue perfusion may be understood by applying Poiseuille’s law (F = πr4ΔP / 8ηL) to capillary blood blow within a muscle compartment. In this equation, F represents capillary blood flow, r is the radius of the capillary to the fourth power, and ΔP is the pressure gradient from the precapillary arteriole to the postcapillary venule. Increasing ICP alters two variables in this equation, ΔP and r. As ICP rises, pressure is transmitted to the postcapillary venules, increasing the venous pressure and decreasing the arterial-venous pressure gradient (ΔP). Furthermore, increased ICP may collapse capillaries, decreasing their radius and further increasing resistance to flow.1,2
Compartment Pressures
Several theories exploring the role of ICP in the development of compartment syndrome have been proposed. The concept of a critical closing pressure was one of the earliest concepts, whereas more recent research suggests that the dynamic ICP threshold more accurately predicts which muscle compartments will develop compartment syndrome.
Critical Closing Pressure
Matsen suggested that there is a “critical closing pressure” above which capillaries collapse from transmural pressure and blood flow is arrested.1 The pressure at which capillary blood flow ceases has been debated over the decades. Using wick catheters inserted into the anterolateral compartment of dogs, Hargens et al demonstrated that baseline capillary hydrostatic pressure in normotensive dogs was 25 mm Hg, whereas hydrostatic pressure in postcapillary venules was 16 mm Hg.2 Hargens proposed that capillary perfusion pressure would drop precipitously if ICP exceeded 30 mm Hg. Using vital microscopy to observe the response of isolated rat cremasteric muscle to increased external pressure, Hartsock found that a pressure gradient between the ICP and mean arterial pressure (MAP) of 25.5 ± 14 mm Hg arrested capillary blood flow. Hartsock saw no significant collapse of arterioles, capillaries, or venules.3 Another study saw no significant collapse of arterioles with increased ICP, but a modest reduction (≤25%) in the diameter of venules.4 Together these studies disproved the “critical closing theory” proposed by Matsen and suggested instead that the arterial-venous pressure gradient (ΔP) is the critical determinant of capillary blood flow.1,3,4 This conclusion has direct implications for determining the threshold ICP that defines compartment syndrome.
Absolute ICP Threshold
Defining the threshold ICP that produces tissue injury and cell death is an important step in determining the pressure at which fasciotomy is advisable. Hargens found that an absolute ICP of 30 mm Hg for 8 hours universally produced muscle necrosis in normotensive dogs, whereas pressures less than 30 mm Hg produced no muscle necrosis.5 Tissues differed in their susceptibility to increased ICPs. Early signs of epineurial venule compression were observed in experimental models at 20-30mm Hg.6,7 The differential susceptibility to injury between tissues may provide an explanation for those cases in which a delayed fasciotomy fails to restore full neurologic function despite viable muscle in the compartment.
Dynamic ICP Threshold
Defining compartment syndrome based on an absolute pressure threshold is appealing in its simplicity but ignores the role of arterial blood pressure in affecting compartmental blood flow. Changes in arterial pressure affect the arterial-venous pressure gradient (ΔP), altering compartment blood flow. Some authors have proposed defining compartment syndrome using a pressure threshold relative to mean arterial pressure (MAP) or diastolic pressure. Heppenstall found that healthy muscle in dogs developed evidence of tissue ischemia on 31P-magnetic resonance spectroscopy when the difference between MAP and ICP (MAP − ICP) dropped below 30 mm Hg.8 Injured muscle showed greater sensitivity to ischemia, as tissue ischemia became evident when the difference between mean arterial pressure and ICP was less than 40 mm Hg [(MAP − ICP) <40 mm Hg].
In a small series of patients, Heppenstall found that a dynamic pressure threshold—(MAP − ICP) <40 mm Hg—prevented unnecessary fasciotomy in a number of patients with absolute ICP exceeding 30 mm Hg.8 Using the diastolic blood pressure as their reference point in a dog study, Heckman found a dramatic increase in tissue injury and necrosis when the difference between the diastolic blood pressure and ICP was less than 10 mm Hg.9 Comparing ICP criteria, McQueen et al found that an absolute ICP threshold of 30 mm Hg would have necessitated fasciotomy in 43% of patients, whereas a dynamic ICP threshold of 30 mm Hg less than diastolic pressure resulted in only three fasciotomies.10 These studies provide compelling data that a dynamic ICP threshold relative to mean arterial pressure or diastolic pressure is more appropriate for selecting patients for fasciotomy.
Clinical Etiologies
Vascular Causes
ICP is elevated by conditions that either increase compartment volume or produce external compression on the compartment. The most common vascular etiologies for compartment syndrome are ischemia-reperfusion (IR) injury associated with acute ischemia, arterial and venous traumatic injuries, crush injuries, phlegmasia cerulea dolens, and hemorrhage within a compartment.
Ischemia-Reperfusion
Compartment syndrome may complicate up to 21% of cases of acute ischemia.11,12 The IR phenomenon, described in detail in Chapter 7, is believed to play a central role in the pathogenesis of compartment syndrome due to acute ischemia and crush injury. IR increases compartment volume by causing muscle tissue injury that results in tissue and interstitial edema. With increasing duration and extent of ischemia, increased microvascular permeability permits the efflux of plasma proteins and progressive interstitial edema.13 With reperfusion, oxygen radical generation causes lipid peroxidation of cell membranes, further augmenting microvascular permeability and exacerbating interstitial edema.13 In a series of 194 fasciotomies performed for acute arterial occlusion, Papalambros identified several risk factors for compartment syndrome after acute arterial ischemia, including prolonged ischemia time (>6 hours), young age, insufficient arterial collaterals, acute time course for arterial occlusion, hypotension, and poor back-bleeding from the distal arterial tree at embolectomy.11
Trauma
Both arterial and venous trauma may produce compartment syndrome. Occlusive arterial injuries result in distal ischemia that initiates the IR phenomenon, whereas venous injuries may compromise venous outflow. The impact of compromised venous outflow is discussed in the following section. The incidence of fasciotomy for trauma varies from 11.3% for blunt trauma to 28% for penetrating vascular trauma.12,14 The need for fasciotomy also varies according to the type of vascular injury. In one series, fasciotomy was performed for 29.5% of isolated arterial injuries, 15.2% of isolated venous injuries, and 31.6% of combined arterial and venous injuries. Injuries to the popliteal artery are notorious for a higher risk of requiring fasciotomy (61% incidence) compared with injuries above the knee (19% incidence).14
Venous Outflow Obstruction
Conditions that dramatically impede venous outflow may predispose a limb to compartment syndrome. Examples include phlegmasia cerulea dolens and harvesting of the deep veins of the thigh. Uncomplicated deep venous thrombosis (DVT) often increases ICP, depending on the extent of DVT, but compartment syndrome is rare in the absence of phlegmasia cerulea dolens.15 The extensive DVT of phlegmasia dramatically impairs venous outflow to produce profound tissue swelling. With increased venous hypertension, the arterial-venous pressure gradient (ΔP) is altered and capillary blood flow is impaired. With reduced capillary blood, muscle cell injury ensues, exacerbating tissue edema. This cycle continues until eventually the postcapillary venules thrombose and venous gangrene develops.
Iatrogenic interruption of venous outflow, such as harvesting of femoral vein for use as conduit for arterial reconstruction, has been associated with the development of compartment syndrome in 17.8% of limbs.16
Nonvascular Etiologies
Fracture
Tibial or forearm fractures are the most common orthopaedic causes of acute compartment syndrome. These fractures injure the surrounding muscles and cause bleeding within the compartment, elevating ICP. The incidence of compartment syndrome with fractures ranges from 1% to 29%.10,19–21 The anterior compartment of the leg and the flexor compartment of the forearm are most prone to this phenomenon. Comminuted fractures are more likely to result in a compartment syndrome, owing to the greater energy absorbed in such injuries.20
Crush Injury
Crush injuries are another form of trauma that may cause compartment syndrome. Reported mechanisms include prolonged immobility due to alcohol or drug intoxication, pinning of a victim beneath heavy equipment at industrial sites or large building structures during earthquakes, and blunt trauma due to assault.22,23 In such cases, compartment syndrome results from direct compartment pressure with underlying muscle ischemia. Upon removal of the external compressive force, the muscle is reperfused, resulting in interstitial edema. Depending on the magnitude of the crush injury, these patients may also require a large resuscitation, because of the volume of fluid sequestered in the interstitium of the crushed muscle, which can exacerbate the increase in ICP.22 Rhabdomyolysis frequently complicates crush injuries and carries a 4% to 33% risk for acute renal failure.24
Iatrogenic
Iatrogenic causes of compartment syndrome include extravasation of large volumes of fluid within a muscle compartment, extravasation of caustic medications such as contrast agents, inadvertent arterial injections, and hemorrhage related to arterial or venous punctures in coagulopathic or anticoagulated patients.25–27 Additional mechanisms include the compression injuries associated with prolonged intraoperative immobilization, as occurs in the dorsal lithotomy position, and cast immobilization for fractures.28,29
Secondary Compartment Syndrome
Rarely, a compartment syndrome develops in a trauma patient without overt evidence of extremity trauma. This phenomenon has been termed “secondary compartment syndrome” and is believed to be a result of a combination of diffuse microvascular permeability due to trauma-induced systemic inflammatory response syndrome in concert with massive fluid resuscitation.30 It is a local manifestation of a systemic illness that may rarely necessitate compartment decompression.
Clinical Presentation
History and Examination
The diagnosis of an acute compartment syndrome begins with a high index of suspicion. Symptoms of a compartment syndrome include pain that is disproportionate to the magnitude of the injury and paresthesias in the distal extremity.31 The pain is typically not relieved by immobilization or reduction of fractures and responds poorly to analgesic medications. Paresthesias represent an early symptom of ischemia of the nerves traversing the muscle compartment in question.
On examination, the most common findings are a tense, swollen compartment with pain elicited by passive movement of the muscles in that compartment. A careful neurologic examination should document sensory and motor function distal to the compartment, focusing especially on the nerves that traverse the compartment at risk. For example, a compartment syndrome afflicting the anterior compartment of the lower leg may be accompanied by dysfunction of the deep peroneal nerve, causing numbness at the first dorsal webspace of the foot or inability to extend the great toe. Loss of two-point discrimination is a sensitive indicator of developing compartment syndrome.32
Clinical examination, including a pulse examination, should also exclude ongoing ischemia since ischemia must be rectified expeditiously. The sensitivity of examination findings for compartment syndrome is dubious, however. In a meta-analysis of compartment syndromes related to tibial fractures, Ulmer found that the sensitivity of clinical findings for diagnosing compartment syndrome is low (13%-19%). The positive predictive value is equally low (11%-15%), and the negative predictive value is high (97%-98%). Ulmer concluded that the “clinical features of compartment syndrome are more useful in their absence in excluding the diagnosis.” Laboratory findings such as creatinine phosphokinase may indicate muscle ischemia or injury, but an elevated creatinine phosphokinase is generally not helpful in diagnosing a compartment syndrome.33
Measurement of Compartment Pressures
Measurements of ICP are not required to ascertain the diagnosis of a compartment syndrome in most cases. Pressure measurement should be reserved for equivocal cases, unconscious patients, and pediatric patients in whom a compartment syndrome is suspected. In cases in which the diagnosis is apparent from history or examination, measuring ICP is superfluous and risks delaying definitive therapy.
Technique
Measuring ICP requires an instrument for measuring compartment pressures and a working knowledge of the muscle compartments of the limb in question. ICP has been measured using a host of techniques and instruments, including simple manometers, wick catheters, slit catheters, side-ported needles, and fiberoptic transducers. Each system has these two components: (1) a needle to access the compartment and (2) a pressure measurement system. The most commonly used ICP measuring systems are the arterial line manometer, handheld Stryker system, and Whiteside manometer. The arterial line manometer is ideally suited for use in the intensive care unit and operating room where pressure transducers and monitors are readily available. In the emergency department, the handheld Stryker system is particularly convenient. In environments in which neither approach is feasible, the Whiteside’s manometer technique (Fig. 163-1) offers a simple, inexpensive alternative.34
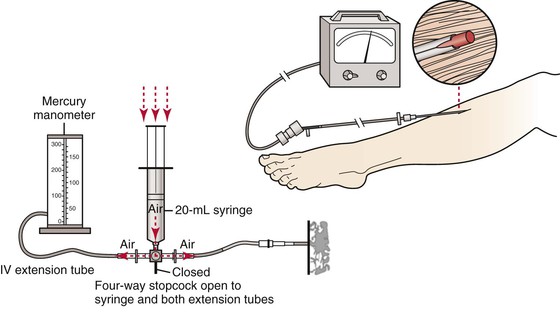
Figure 163-1 Whiteside’s technique for measuring ICP. Essential equipment includes a sterile 18-gauge (1.25-inch) needle, sterile intravenous (IV) extension tubing, a four-way stopcock, a second section of IV extension tubing, and blood pressure manometer that are connected sequentially. A 20-mL syringe filled with 15 mL of air is connected to the four-way stopcock. The syringe is inserted into a bag of sterile saline, and saline is aspirated until it fills approximately half of the first length of IV extension tubing. The stopcock is turned off, and the needle is inserted into the muscle compartment of interest, using aseptic technique. The stopcock is turned so that both sections of the extension tubing are open to the syringe. Air is gently injected to the point that the saline meniscus in the tube begins to move. The pressure at which the saline meniscus just begins to move represents the compartment pressure.
The most comprehensive comparison of the accuracy of the various ICP measurement devices was performed by Boody using a graduated cylinder to generate a known pressure.34 Boody found that side-port needles and slit catheters were more accurate than straight needles. Straight needles tended to overestimate pressure compared with the other two access systems. The arterial line manometer and handheld Stryker device were more accurate than the Whiteside’s manometer as pressure measurement systems. For that reason, the author favors an arterial line manometer or handheld Stryker device.
The choice of compartments for pressure measurement should be guided by clinical examination. The most symptomatic or turgid compartment should be interrogated first, remembering that the anterior compartment of the lower leg and the flexor compartment of the forearm are most prone to compartment syndrome. Ultimately it is advisable to obtain compartment pressures from all compartments at risk. If necessary, multiple readings may be obtained. ICP, mean arterial pressure, and diastolic pressure should be recorded for the medical record. The pressure criteria that define a compartment syndrome will be discussed later in this section. A normal compartment pressure is ≤10 to 12 mm Hg.
Alternative Objective Techniques
Near-infrared spectroscopy and laser Doppler flowmetry have been proposed as noninvasive techniques to aid in identifying an evolving compartment syndrome.35–37 A paucity of clinical data exists to validate these techniques, and the relative unavailability of these technologies has limited their utility.
Unusual Presentations for Compartment Syndrome
Compartment syndrome may affect any myofascial compartment. Although less common than the lower leg, compartment syndromes have been described in the upper extremity, hand, thigh, foot, and buttock. For any of these compartment syndromes, pain out of proportion to examination and swelling remain the hallmarks of their clinical presentation. In some cases, pain with passive motion may exacerbate the pain. The presence of neurologic symptoms is highly variable and is not required to secure the diagnosis.
Hand compartment syndromes are usually associated with crush injuries or fractures of the carpal bones. Hand compartment syndrome may affect any of the 10 compartments of the hand. The classic symptoms are pain and local paralysis at the intrinsic muscles.38 Forearm compartment syndromes are typically associated with direct blows, crush injuries, or distal radius and ulnar fractures.39 Pain, swelling, and neurologic symptoms are the classic symptoms of increased ICP in the forearm, especially after trauma.39
Thigh compartment syndrome is usually caused by blunt trauma from motor vehicle accidents, contusion,40 or crush injury,22 although bleeding complications in the thigh17,18 and exercise-induced injuries41 have been implicated. Reperfusion injury rarely results in a compartment syndrome of the thigh. The anterior thigh compartment is most commonly involved and universally presents with pain on passive motion. Paresthesias and paralysis may also be present.40
Gluteal compartment syndrome has been associated with hypogastric artery ligation or embolization during aortic aneurysm repair, hip arthroplasty, and prolonged compression during operative procedures.42–45 Gluteal compartment syndrome has been cited as a cause of rhabdomyolysis, renal failure, and sciatic nerve palsy.42
Adjunctive Measures
Prevention of Compartment Syndrome
A variety of adjuncts have been proposed to mitigate muscle swelling and prevent the development of compartment syndrome. The most common approach has been pharmacologic therapy to blunt the IR phenomenon. The pharmacologic interventions are aimed primarily at modulating oxygen radical formation during reperfusion of an ischemic limb to minimize IR injury. Mannitol, allopurinol, superoxide dismutase, deferoxamine, thromboxane A2, and melatonin have shown promise in reducing oxygen radical formation and lowering compartment pressures in animal models.46–50 The use of these agents in humans has been limited to anecdotal reports of benefit.51,52
In cases of impending compartment syndrome, Mars53 proposed a protocol of “first aid to hypoxic cells.” Mars’ protocol included (1) maintaining normal blood pressure, since hypotension reduces perfusion pressure; (2) removing any constricting bandages; (3) maintaining the limb at heart level (with no elevation) to avoid reducing the arterial-venous pressure gradient; and (4) providing supplemental oxygen to optimize oxygen saturation. This protocol represents a simple, rational approach to minimizing the risk for compartment syndrome based on its pathophysiology.
Prevention of Systemic Sequelae
Myonecrosis is a recognized complication of compartment syndrome. With myonecrosis, large quantities of intracellular potassium, phosphate, myoglobin, and creatine phosphokinase are liberated. Treatments designed to prevent systemic sequelae of compartment syndrome are aimed at preventing further complications related to the electrolyte disturbances or myoglobinuria that result from extensive myonecrosis.
Hyperkalemia with cardiac arrest has been described among patients with significant myonecrosis, especially among patients with crush injuries.22,23 Treatment of hyperkalemia with oral binding resins, loop diuretics, or insulin and glucose may be inadequate, especially with concurrent acidosis or acute renal failure. Daily hemodialysis or continuous hemofiltration may be required to control hyperkalemia.24
Myoglobinuria exerts its nephrotoxic effects by inducing renal vasoconstriction, tubular cast formation, and direct heme protein-induced cytotoxicity.24 The management of myoglobinuria includes aggressive crystalloid infusion, forced diuresis with mannitol, and alkalinization of the urine with bicarbonate. The rationale for crystalloid and bicarbonate infusion is based on the observation that heme proteins have minimal nephrotoxicity in the absence of hypovolemia and aciduria.24 Clinical data affirm that early resuscitation with crystalloid diminishes the risk of progression to acute renal failure.22–24 The data in support of mannitol and bicarbonate are more circumstantial. In a review of 1771 patients with acute renal failure due to myoglobinuria, Brown et al found no difference in the incidence of acute renal failure, need for dialysis, or mortality between patients receiving mannitol and bicarbonate, compared with those receiving crystalloid infusion alone.54 Current recommendations for the management of myoglobinuria include hydration with a goal urine pH of greater than 6.5, despite the fact that the use of sodium bicarbonate has not been shown to be superior to saline diuresis.55
Myoglobin is poorly cleared by conventional dialysis membranes because of its relatively large molecular weight (17,000 Da), so hemodialysis is not a useful adjunct in preventing renal injury due to myoglobinuria, though continuous venovenous hemofiltration has shown promise in preliminary studies.56 Renal replacement therapy is currently reserved for standard indications, including the management of severe hyperkalemia.55
Fasciotomy
Criteria for Fasciotomy
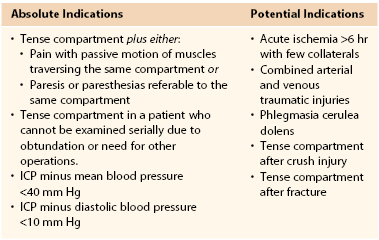
The decision to proceed to fasciotomy may be dictated on clinical grounds with or without ICP measurements (Table 163-1).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree