The standard echocardiographic evaluation of left ventricular (LV) mass, particularly in ischemic cardiomyopathy (IC) is challenging because it is based on geometric assumptions. The aim of this study was to assess the accuracy of LV mass calculation using echocardiographic modalities compared with cardiac magnetic resonance (CMR) in IC and in nonischemic cardiomyopathy (non-IC). Echocardiography was performed in 104 patients (mean age 55 ± 15 years) referred for CMR: 63 with IC and 41 with non-IC. CMR, M-mode echocardiography, 2-dimensional echocardiography, and 3-dimensional echocardiography (3DE) were analyzed using standard commercial tools to obtain LV mass. LV mass on 3DE showed a higher correlation with CMR than 2-dimensional echocardiography (r = 0.87 vs r = 0.70, p <0.001). M-mode echocardiography overestimated LV mass (bias +30%) and 2-dimensional echocardiography underestimated LV mass (bias −11%), whereas measurements on 3DE showed only minimal bias (−2%). LV mass on 3DE in non-IC showed a significantly higher correlation with CMR than in IC (r = 0.92 vs r = 0.84, z = 2.3, p <0.05). In non-IC, the mean difference was −2 g (−1% of the mean), with 95% limits of agreement of ±33 g (±19% of the mean). In IC, the mean difference was −7 g (−4% of the mean), with limits of agreement of ±56 g (±31% of the mean). There was a correlation between the absolute LV mass differences (3DE derived and CMR derived) and scar percentage (infarcted mass/total LV mass) using delayed-hyperenhancement images (r = 0.40, p <0.05). The net reclassification index with 3DE was +16% for concentric LV hypertrophy. In conclusion, the most accurate and reliable echocardiographic measurement of LV mass is 3DE, but underestimation and variability remain challenges in IC.
The measurement of left ventricular (LV) mass is of importance for cardiovascular risk stratification. The severity of LV hypertrophy is an independent risk factor for cardiovascular mortality and morbidity in ischemic cardiomyopathy (IC). Quantitative assessment of LV mass is essential for accurate diagnosis and determination of the severity of LV hypertrophy. Increased LV mass portends increased risk for morbidity and mortality after high-risk myocardial infarction, and hypertrophy regression in patients with hypertension is associated with a reduced risk for events. Echocardiography is the most commonly used imaging modality to evaluate LV mass, using linear (M-mode) echocardiography or 2-dimensional echocardiography (2DE). However, the accuracy of these techniques is limited because of their underlying geometric assumptions about LV shape, which may be inaccurate, particularly in patients with regional changes due to coronary disease. In contrast, 3-dimensional echocardiography (3DE) may allow more accurate and reproducible measurements of LV mass without geometric assumptions and modeling. Although previous studies have described the relation between 3DE-derived LV mass and cardiac magnetic resonance (CMR)–derived LV mass, most have involved limited numbers of patients, few have included patients with LV dysfunction, and the relative impact of IC and non-IC origin on the reliability of LV mass calculation has not been addressed. We hypothesized that the accuracy of LV mass assessment by 3DE may be compromised in the setting of severe ischemic LV dysfunction. The aims of this study were to assess the accuracy and reproducibility of 3-dimensional echocardiographic measurement of LV mass with IC and non-IC and to assess the additional value of 3DE for diagnosis of LV hypertrophy.
Methods
From January 2010 to December 2012, we enrolled 117 consecutive patients referred for CMR imaging to assess myocardial viability. Three-dimensional echocardiography was performed within 30 days of CMR. Exclusion criteria were an LV ejection fraction >50% and frequent arrhythmia (atrial fibrillation or >5 premature beats/min). We excluded 13 patients (11%) because of poor echocardiographic image quality. Therefore, 104 patients were included in the final analysis. Patients with significant coronary artery stenosis (≥50%) in ≥1 epicardial coronary vessel on angiography, revascularization, and/or a history of myocardial infarction were classified as having IC. Patients were classified as having non-IC if they had none of these features. Clinical variables were prospectively gathered from patients and their medical records at the time of echocardiography. The protocol was approved by the Cleveland Clinic Institutional Review Board.
Digitally archived CMR studies were reviewed by experienced independent readers. CMR was performed on commercially available CMR scanners (Achieva 1.5-T XR or Ingenia 3.0-T; Philips Medical Systems, Best, The Netherlands). The time difference between echocardiography and CMR was 7 ± 10 days (range 0 to 30). Electrocardiographically gated localizing spin-echo sequences were used to identify the LV long axis. Steady-state free-precession dynamic gradient-echo cine loops were obtained during 10- to 15-second breath-holds. Data were reconstructed to obtain 25 to 30 frames/cardiac cycle. In all patients, 10 to 12 short-axis cine loops were obtained from the ventricular base to the apex. In every short-axis slice, endocardial and epicardial contours were manually traced at end-diastole, including the papillary muscles and the endocardial trabeculae in the LV cavity. LV mass was determined using the disk-area summation method and was calculated as the difference of volumes multiplied by the specific mass of myocardial tissue (1.05 g/ml). Subsequently, delayed-hyperenhancement (DHE) images were obtained to identify LV scar in the long and short-axis orientations, about 10 to 20 minutes after the injection of 0.2 mmol/kg of gadolinium dimeglumine. DHE CMR images were analyzed using commercially available software (cvi 42 ; Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Endocardial and epicardial myocardial edges were manually delineated on DHE CMR images. Scar was defined by intensity >2 SDs higher than user-defined viable myocardium. The scar percentage was automatically determined as the percentage of total myocardium (infarct mass divided by total LV mass).
Transthoracic echocardiography was performed by experienced sonographers using a commercially available ultrasound machine (Vivid 9, GE Vingmed Ultrasound AS, Horten, Norway; iE33, Philips Medical Systems, Andover, Massachusetts). Standard imaging windows were used, including parasternal long and short axes, as well as apical 4-chamber, 2-chamber, and long-axis views. Echocardiographic images were digitally recorded and downloaded to a server for off-line analysis. M-mode measurements of LV mass were obtained from the parasternal long-axis view. The maximal LV diastolic dimension was used to ensure that wall thickness and chamber diameter measurements were perpendicular to the endocardium at the level of the chordae tendineae. LV internal diameter and interventricular septal and posterior wall thicknesses were measured at end-diastole, and LV mass by 2-dimensionally guided M-mode echocardiography was derived using the American Society of Echocardiography’s guideline formula. Two-dimensional echocardiographic images were obtained from the apical 4- and 2-chamber views, taking care to maximize the LV long-axis dimension. For the 2 apical views, end-diastolic frames were selected, and endocardial and epicardial contours were traced manually, including the papillary muscles in the LV cavity. Points on both sides of the mitral annulus were connected by a straight line. The traced contours were then used to calculate endocardial and epicardial LV volumes using Simpson’s formula. The difference between the epicardial and endocardial volumes was computed for each view and multiplied by the specific mass of myocardial tissue (1.05 g/ml) and averaged for the views to obtain a biplane estimate of LV mass. The volumetric transthoracic echocardiographic data sets were digitally stored and transferred to a workstation with dedicated software platform for off-line postprocessing analysis (Image Arena; TomTec Imaging Systems GmbH, Unterschleissheim, Germany). LV 3-dimensional reconstruction was obtained using the corresponding software modality (4D LV-Analysis version 2.7; TomTec Imaging Systems GmbH) for 3-dimensional echocardiographic acquisitions. The LV epicardial and LV endocardial contours were marked in the apical 4-, 2-, and 3-chamber views and were then tracked automatically frame by frame, starting from end-diastole. Correction of the contours was performed manually if necessary. LV mass was then calculated as LV epicardial volume minus LV endocardial volume multiplied by the specific mass of myocardial tissue (1.05 g/ml) ( Figure 1 ).
The ability to accurately assess LV mass in different LV geometries is important in patients with myocardial infarctions, because mass and geometry influence the risk for adverse cardiovascular events. Gender-specific values of LV hypertrophy were defined as previously: LV mass/height 2.7 >39 g/m 2.7 (women) and >48 g/m 2.7 (men). The classification of concentricity (LV mass/end-diastolic volume) was based on whether concentricity 0.67 was increased (defined as >8.1 g/mL 0.67 in women and >9.1 g/ml 0.67 in men). Patients were divided into 4 morphologies of LV hypertrophy: normal geometry (no LV hypertrophy and normal concentricity), concentric remodeling (no LV hypertrophy and increased concentricity), concentric hypertrophy (LV hypertrophy and increased concentricity), and eccentric hypertrophy (LV hypertrophy and normal concentricity), using previous studies.
Data are reported as mean ± SD. Agreement between each technique (M-mode echocardiography, 2DE, 3DE, and CMR) is expressed using Pearson’s correlation coefficients. Bland-Altman analysis was used to determine the bias and 95% limits of agreement (LOA) between the echocardiographic and CMR measurements. Z transformations were performed to examine differences between correlations between each group. Receiver-operating characteristic curves were generated using MedCalc version 12.3.0 (MedCalc Software, Mariakerke, Belgium). The best cutoff value was defined as the point with the highest sum of sensitivity and specificity. A net reclassification index was calculated for comparison of measurements. The sample size calculation was based on the assumption that the r value of the correlation for LV mass between CMR and 3DE should have a minimum value of 0.82, according to previous studies. The minimum sample size in each group was calculated to be 18 (power 0.90). Inter- and intraobserver variability was examined for M-mode, 2-dimensional, and 3-dimensional echocardiographic data. Measurements were performed in a group of 15 randomly selected subjects by 1 observer then repeated on 2 separate days by 2 observers who were unaware of the other’s measurements and of the study time point. Inter- and intraobserver percentage variability was expressed as the absolute value of the difference between measurements divided by the mean of the measurements. Statistical analysis was performed using SPSS version 20.0 (SPSS, Inc., Chicago, Illinois), and significance was defined as p <0.05.
Results
Echocardiographic and CMR imaging was performed in 104 patients (63 with IC and 41 with non-IC; Table 1 ). Inter- and intraobserver variability is reported in Table 2 .
| Variable | Value |
|---|---|
| Age (yrs) | 54 ± 14 |
| Men | 78 (75%) |
| Body surface area (m 2 ) | 1.9 ± 0.2 |
| Diagnosis | |
| IC | 63 (61%) |
| Non-IC | 41 (39%) |
| LV volumetric data by CMR | |
| LVEDVi (ml) | 135 ± 45 (54–245) |
| LVESVi (ml) | 95 ± 40 (38–193) |
| LVEF (%) | 31 ± 12 (6–50) |
| LV mass/height 2.7 (g/m 2.7 ) | 50 ± 12 (25–87) |
| Type of LV hypertrophy ∗ by CMR | |
| None (no hypertrophy, normal concentricity) | 37 (35%) |
| Concentric remodeling (no hypertrophy, increased concentricity † ) | 3 (3%) |
| Concentric hypertrophy (LV hypertrophy, increased concentricity † ) | 34 (33%) |
| Eccentric hypertrophy (LV hypertrophy, normal concentricity) | 30 (29%) |
∗ LV mass/height 2.7 >39 g/m 2.7 in women and >48 g/m 2.7 in men.
† Concentricity was increased (defined as >8.1 g/ml 0.67 in women and >9.1 g/ml 0.67 in men).
| Modality | Intraobserver | Interobserver |
|---|---|---|
| M-mode echocardiography | 7.4 ± 4.6% | 9.1 ± 4.5% |
| 2DE | 8.8 ± 5.9% | 13.6 ± 8.4% |
| 3DE | 6.1 ± 5.0% | 8.7 ± 5.1% |
The mean CMR-derived LV mass was 185 ± 43 g (range 102 to 344). The mean M-mode echocardiographic LV mass was 264 ± 79 (range 90 to 439), which was overestimated, although there was modest correlation with CMR (r = 0.55, p <0.001). Bland-Altman analysis showed a mean difference of +79 g (+30% of the mean), with LOA of ±129 g (±49% of the mean) ( Figure 2 ). The mean 2DE-derived LV mass was 166 ± 37 g (range 71 to 266 g). A good correlation was noted with CMR (r = 0.70, p <0.001). Bland-Altman analysis showed a mean difference of −18 g (−11% of the mean), with LOA of ±64 g (±39% of the mean) ( Figure 2 ). The mean 3DE-derived LV mass was 181 ± 51 g (range 62 to 327). The correlation with CMR was also good (r = 0.87, p <0.001), but with less bias; Bland-Altman analysis showed a mean difference of −3 g (−2% of the mean), with LOA of ±49 g (±27% of the mean) ( Figure 2 ). LV mass on 3DE had a higher correlation with CMR than with 2DE (p <0.001).
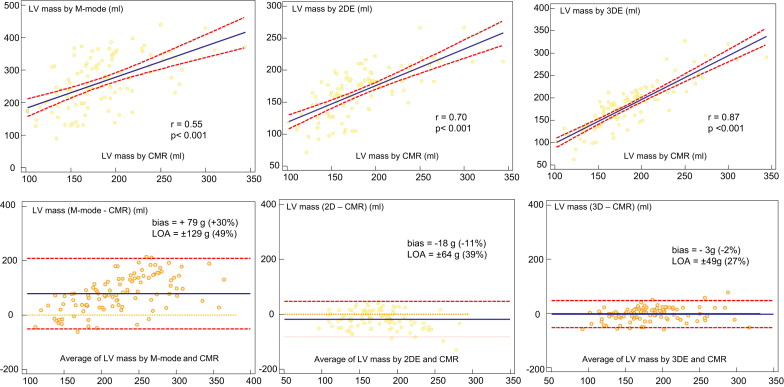
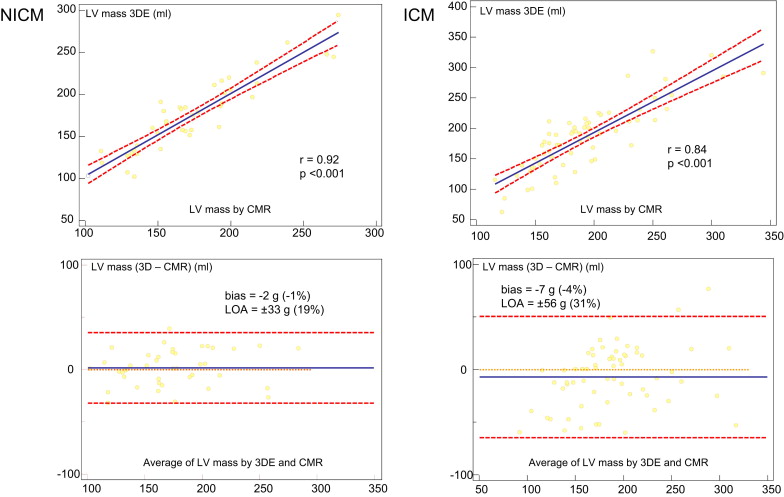
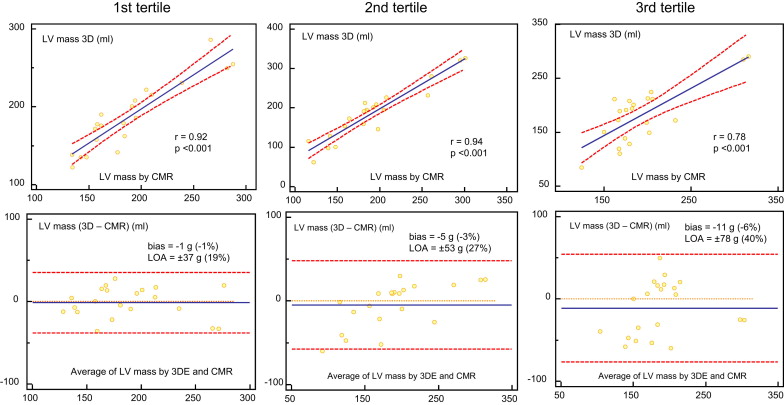
Figure 3 shows the results of the comparison between 3DE and CMR in patients with IC and those with non-IC. In patients with non-IC, Bland-Altman analysis showed a mean difference of −2 g (−1% of the mean), with LOA of ±33 g (±19% of the mean). In patients with IC, Bland-Altman analysis showed a mean difference of −7 g (−4% of the mean), with LOA of ±56 g (±31% of the mean). LV mass on 3DE in patients with non-IC showed a significantly higher correlation with CMR than in those with IC (r = 0.92 vs r = 0.84, p <0.05). In addition, we assessed the correlations of both measurements according to the tertile of infarct size using the scar percentage on DHE CMR (average value 16 ± 10%; Figure 4 ). In the first tertile (mean scar percentage 6 ± 3%), Bland-Altman analysis showed a mean difference of −1 g (−1% of the mean), with LOA of ±37 g (±19% of the mean). In the second tertile (mean scar percentage 15 ± 3%), Bland-Altman analysis showed a mean difference of −5 g (−3% of the mean), with LOA of ±53 g (±27% of the mean). In the third tertile (mean scar percentage 28 ± 7%), Bland-Altman analysis showed a mean difference of −11 g (−6% of the mean), with LOA of ±78 g (±40% of the mean). In patients with IC, there was a weak but significant correlation between the absolute LV mass differences (3DE minus CMR) and the scar percentage (r = 0.40, p <0.05).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


