The outcomes of hemodynamic support during high-risk percutaneous coronary intervention in the very elderly are unknown. We sought to compare outcomes between the patients ≥80 years versus patients <80 years enrolled in the PROTECT II (Prospective Randomized Clinical Trial of Hemodynamic Support with the Impella 2.5 versus Intra-Aortic Balloon Pump in Patients undergoing High Risk Percutaneous Coronary Intervention) randomized trial. Patients who underwent high-risk percutaneous coronary intervention with an unprotected left main or last patent conduit and a left ventricular ejection fraction ≤35% or with 3-vessel disease and a left ventricular ejection fraction ≤30% were randomized to receive an intra-aortic balloon pump or the Impella 2.5; 90-day (or the longest follow-up) outcomes were compared between patients ≥80 years (n = 59) and patients <80 years (n = 368). At 90 days, the composite end point of major adverse events and major adverse cerebral and cardiac events were similar between patients ≥80 and <80 years (45.6% vs 44.1%, p = 0.823, and 23.7% vs 26.8%, p = 0.622, respectively). There were no differences in death, stroke, or myocardial infarction rates between the 2 groups, but fewer repeat revascularization procedures were required in patients ≥80 years (1.7% vs 10.4%, p = 0.032). Bleeding and vascular complication rates were low and comparable between the 2 age groups (3.4% vs 2.4%, p = 0.671, and 6.8% vs 5.4%, p = 0.677, respectively). Multivariate analysis confirmed that age was not an independent predictor of major adverse events (odds ratio = 1.031, 95% confidence interval 0.459–2.315, p = 0.941), whereas Impella 2.5 was an independent predictor for improved outcomes irrespective of age (odds ratio = 0.601, 95% confidence interval 0.391–0.923, p = 0.020). In conclusion, the use of percutaneous circulatory support is reasonable and feasible in a selected octogenarian population with similar outcomes as those of younger selected patients. Irrespective of age, the use of Impella 2.5 was an independent predictor of favorable outcomes.
Complex and high-risk percutaneous coronary interventions (PCIs) are being performed more frequently by interventional cardiologists. Mechanical hemodynamic support has become a frequently used adjunctive tool in the very high-risk patients who commonly present with a combination of poor left ventricular ejection fraction (LVEF), heart failure, and complex coronary anatomy. There is no evidence that these devices are safe in the very elderly considering the potential risk for vascular complications and left ventricular injury, including perforation. PROTECT II (Prospective Randomized Clinical Trial of Hemodynamic Support with the Impella 2.5 versus Intra-Aortic Balloon Pump in Patients undergoing High Risk Percutaneous Coronary Intervention) was a randomized trial that enrolled patients who presented with high-risk co-morbidities, complex anatomy, and who were deemed to require elective hemodynamic support before PCI. In this report, outcomes in the subset of patients ≥80 years enrolled in the PROTECT II trial were compared with a <80-year-old group participating in the study.
Methods
PROTECT II was a prospective, multicenter randomized trial conducted in the United States, Canada, and Europe. The study design, end points, and outcomes have been previously reported. Patients from 112 sites who underwent high-risk PCI and deemed to require circulatory support were randomized to treatment with the Impella 2.5 device versus the intra-aortic balloon pump (IABP) to assess whether PCI with the Impella 2.5 device would result in better outcomes than PCI with IABP support. Patients enrolled in the study were scheduled to undergo a nonemergency PCI on an unprotected left main or last patent coronary vessel with an LVEF ≤35% or PCI in patients with 3-vessel disease and an LVEF ≤30%. Operators were asked to aim for the most complete revascularization possible based on the myocardium at risk in a single procedure. Major exclusion criteria included documented acute myocardial infarction (MI) or persistent elevation of cardiac enzymes, severe peripheral vascular disease, and age >90 years. The study protocol was reviewed and approved by the Ethics Review Committee of each participating center, and all patients provided written informed consent before enrollment.
The primary objective of this analysis was to evaluate outcomes between patients ≥80 and <80 years. The secondary objective was to evaluate the safety trends between the 2 devices (Impella 2.5 vs IABP). Outcomes were evaluated based on the same end points as the PROTECT II trial end points. The primary end point of the study was a composite of 10 individual major adverse event (MAE) components ranging from death to angiographic failure. For this post hoc analysis, the composite MAE end point was the same. In addition, we report 2 additional composites of major adverse cerebral and cardiac events (MACCEs) retrospectively (defined as MACCE 1 and MACCE 2) that included death, stroke, MI, and repeat revascularization. The composites of MACCE 1 and MACCE 2 of the PROTECT II were previously defined. Although both composites included death, stroke, MI, and repeat revascularization as components, MACCE 1 included a threshold of ≥3× upper limit of normal (ULN) for cardiac biomarkers release for the diagnosis of periprocedural MI (defined at the time of the trial design), whereas MACCE 2 used a threshold of ≥8× ULN to reflect a more contemporary and clinically relevant definition of periprocedural MI. All outcomes are reported at 90 days, the longest available follow-up in the study.
Data collection, management and monitoring, events adjudication, and statistical analyses were conducted by Harvard Clinical Research Institute (Boston, MA). An independent Clinical Events Committee blinded to the treatment group assignment adjudicated all study end points. Echocardiographic and angiographic end points were adjudicated independently by 2 academic center core laboratories (Duke Clinical Research Institute, Durham, NC, and Beth Israel Deaconess, Boston, MA, respectively).
The data were expressed as mean ± SD, median (range), or proportions as appropriate. A univariate parametric analysis was performed using a 2-tailed unpaired t test or a nonparametric Mann-Whitney test for continuous outcomes. Pearson’s chi-square test or Fisher’s exact tests were used as appropriate for nominal data. A one-way analysis of variance was performed to compare baseline and procedural characteristics between patient groups. Kaplan-Meier estimates of the cumulative incidence of MAE were calculated, and a log-rank test was performed to compare the clinical outcomes between the groups. A logistic multivariate analysis was used to select the independent predictors of outcomes in the overall patient population while adjusting for age (≥80 vs <80 years) and device. The logistic model included all procedural or baseline variables that were significantly different (p <0.05) in the univariate analysis between the 2 age groups. The interaction between the Impella 2.5 device and age (≥80 vs <80 years) was also included in the model to examine the safety of Impella 2.5 with respect to the prevalence of adverse event rates across the age groups. All p values were 2 tailed and considered significant when the probability is <0.05. The statistical analyses for this report were performed by the Harvard Clinical Research Institute using Statistical Analysis System, version 9.2 (SAS Institute Inc, Cary, NC).
Results
This analysis includes all patients who met the study eligibility criteria and enrolled in the PROTECT II (n = 427). Patients ≥80 years represented 13.8% of the total population (n = 59). Demographics and baseline characteristics comparing the patients ≥80 years and the patients <80 years are presented in Table 1 . The mean age of the patients ≥80 years was 83.5 ± 3 years (range 80–90 years). Patients ≥80 years had lower body mass index, were more anemic, had more renal insufficiency, and presented more frequently with an acute coronary syndrome or a non–ST-segment elevation MI with elevated biomarkers than the younger group cohort. Their predicted rate of mortality using both the logistic Euroscore and the Society of Thoracic Surgery scores were also significantly higher. The procedural and angiographic characteristics of the 2 groups are presented in Tables 2 and 3 . The coronary disease burden and the extent of revascularization were similar between the 2 groups as assessed by the SYNTAX score and ischemic zone score. Left main trunk lesions were attempted more often in patients ≥80 years than in patients <80 years (17.1% vs 9.9%, p = 0.009). The number of patients with severe calcification was significantly higher in patients ≥80 years than in patients <80 years (30.1% vs 14.7%, p <0.001). Consequently, rotational atherectomy was used more frequently in the patients ≥80 years (22.2% vs 10.6%, p = 0.013). Angiographic complications were similar between groups except there was more distal embolization encountered in patients ≥80 years compared with patients <80 years.
| Patient Characteristic | Patients With Age <80 Yrs (n = 368) | Patients With Age ≥80 Yrs (n = 59) | p-Value |
|---|---|---|---|
| Age, mean ± SD (male) | 64.6 ± 9.3 | 83.5 ± 3.0 | <0.001 |
| Male | 82.1% | 76.3% | 0.290 |
| Weight (lbs) | 187.7 ± 44.6 | 159.2 ± 29.0 | <0.001 |
| Height (in) | 67.9 ± 3.6 | 66.5 ± 3.7 | 0.008 |
| Prior myocardial infarction | 68.4% | 64.4% | 0.543 |
| Angina pectoris | 66.4% | 70.7% | 0.518 |
| Stable | 62.6% | 43.9% | 0.024 |
| Unstable | 37.4% | 56.1% | 0.024 |
| Heart failure | 86.1% | 93.2% | 0.132 |
| Arrhythmia | 47.3% | 61.0% | 0.050 |
| Percutaneous coronary intervention | 41.3% | 28.8% | 0.070 |
| Coronary bypass | 35.6% | 25.4% | 0.126 |
| Peripheral vascular disease | 26.8% | 22.0% | 0.435 |
| Prior stroke | 14.4% | 15.3% | 0.863 |
| Diabetes mellitus | 53.0% | 40.7% | 0.079 |
| Hypertension | 86.4% | 86.4% | 0.995 |
| Chronic obstructive lung disease | 26.6% | 33.9% | 0.243 |
| Renal insufficiency | 23.7% | 42.4% | 0.003 |
| History of tobacco use | 73.8% | 47.4% | <0.001 |
| Current tobacco user | 49.3% | 7.4% | <0.001 |
| Left ventricular ejection fraction | 23.55 ± 6.23 | 24.68 ± 6.84 | 0.203 |
| Additive EuroScore | 7.98 ± 3.33 | 13.17 ± 9.54 | <0.001 |
| Logistic EuroScore | 15.88 ± 15.13 | 35.34 ± 22.27 | <0.001 |
| STS mortality score | 5.03 ± 5.80 | 11.80 ± 7.94 | <0.001 |
| STS mortality/morbidity score | 27.57 ± 14.62 | 41.90 ± 15.12 | <0.001 |
| Acute MI at admission | 9.2% | 27.1% | <0.001 |
| Blood urea nitrogen (mg/dL) | 22.01 ± 12.13 | 26.39 ± 15.32 | 0.040 |
| Serum creatinine (mg/dL) | 1.21 ± 0.50 | 1.36 ± 0.66 | 0.088 |
| Hemoglobin (g/dL) | 12.77 ± 1.93 | 11.81 ± 1.80 | <0.001 |
| Hematocrit (%) | 37.96 ± 5.39 | 35.28 ± 4.99 | <0.001 |
| Creatine kinase (U/L) | 78.23 ± 66.92 | 63.34 ± 37.30 | 0.015 |
| Creatine kinase MB (ng/mL) | 2.39 ± 1.89 | 2.83 ± 2.98 | 0.297 |
| Troponin (ng/mL) | 0.50 ± 1.73 | 1.25 ± 3.37 | 0.097 |
| Procedural Characteristic | Patients With Age <80 Yrs (n = 368) | Patients With Age ≥80 Yrs (n = 59) | p Value |
|---|---|---|---|
| Number of lesions treated | 2.87 ± 1.46 | 2.88 ± 1.47 | 0.965 |
| Number of stents placed | 2.99 ± 1.83 | 2.98 ± 1.75 | 0.981 |
| Total length of all lesion treated (mm) | 36.44 ± 26.91 | 31.32 ± 24.45 | 0.170 |
| Use of RA during index procedure | 10.6% | 22.0% | 0.013 |
| Contrast administered during PCI (cm 3 ) | 257.61 ± 132.80 | 234.12 ± 108.83 | 0.198 |
| Duration of index procedure (hour) | 1.06 ± 0.70 | 0.92 ± 0.51 | 0.075 |
| Device support ≥3 hours post PCI | 15.2% | 25.4% | 0.049 |
| Patients discharged from Cath lab on device support | 20.2% | 28.8% | 0.136 |
| Transfusion was required during PCI or at pump removal | 2.4% | 3.4% | 0.671 |
| Number of units transfused during the procedure or at pump removal | 2.11 ± 1.17 | 2.50 ± 2.12 | 0.712 |
| Any of hematoma >5, blood loss at vascular site access requiring additional treatment, or retroperitoneal bleed | 5.4% | 6.8% | 0.677 |
| Subject transferred to ICU after the procedure | 77.1% | 81.4% | 0.467 |
| Heparin administered during procedure | 89.1% | 81.4% | 0.089 |
| IIb/IIIa Inhibitors used at baseline | 19.3% | 23.7% | 0.428 |
| SVG interventions | 11.7% | 6.8% | 0.264 |
| Syntax score Pre-PCI | 29.82 ± 13.34 (242) | 30.43 ± 14.11 (44) | 0.781 |
| Syntax score Post-PCI | 14.71 ± 12.80 (241) | 14.90 ± 12.32 (44) | 0.927 |
| Syntax score Post-PCI – Pre-PCI | −15.14 ± 9.43 (241) | −15.53 ± 9.63 (44) | 0.801 |
| Ischemia zone score Pre-PCI | 8.77 ± 2.14 (342) | 9.28 ± 1.86 (57) | 0.092 |
| Ischemia zone score Post-PCI | 4.26 ± 3.01 (340) | 4.46 ± 3.17 (57) | 0.654 |
| Gain in ischemia zone score | 4.51 ± 2.77 (340) | 4.82 ± 3.18 (57) | 0.441 |
| Lesion Characteristic | Patients With Age <80 Yrs (n = 368 Patients n = 932 Lesions) | Patients With Age ≥80 Yrs (n = 59 Patients n = 146 Lesions) | p Value |
|---|---|---|---|
| Vessel location | |||
| LAD | 18.7% | 16.4% | 0.517 |
| Left main | 9.9% | 17.1% | 0.009 |
| LCx | 37.1% | 30.8% | 0.141 |
| RCA | 28.0% | 32.2% | 0.298 |
| SVG | 6.3% | 3.4% | 0.167 |
| Calcification | |||
| None/mild | 64.7% | 45.9% | <0.001 |
| Moderate | 20.5% | 24.0% | 0.343 |
| Severe | 14.7% | 30.1% | <0.001 |
| Total occlusion | 7.0% | 2.1% | 0.023 |
| TIMI flow | |||
| 0 | 4.2% | 0.7% | 0.037 |
| 1 | 2.8% | 1.4% | 0.314 |
| 2 | 4.4% | 4.1% | 0.869 |
| 3 | 88.6% | 93.8% | 0.057 |
| Bifurcation | 34.9% | 39.7% | 0.262 |
| Thrombus | 0.0% | 0.0% | — |
| Aneurysm | 1.7% | 2.1% | 0.785 |
| Final TIMI flow | |||
| 0 | 1.6% | 0.7% | 0.385 |
| 1 | 0.4% | 0.7% | 0.679 |
| 2 | 0.8% | 0.0% | 0.291 |
| 3 | 97.2% | 98.6% | 0.309 |
| No reflow | 1.5% | 2.1% | 0.629 |
| Transient | 71.4% | 100.0% | 0.290 |
| Sustained | 28.6% | 0.0% | 0.290 |
| Abrupt closure | 1.2% | 1.4% | 0.855 |
| Transient | 72.7% | 100.0% | 0.400 |
| Sustained | 27.3% | 0.0% | 0.400 |
| Perforation | 0.7% | 0.7% | 0.961 |
| Spasm | 0.1% | 0.0% | 0.691 |
| Distal embolus | 0.3% | 2.1% | 0.009 |
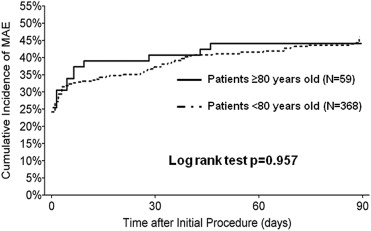
The need and amount of blood transfusion required post-PCI were similar between the 2 groups (p = 0.671 and p = 0.712, respectively). The incidence of large hematomas, blood loss from access site, or retroperitoneal bleeding was also similar ( Table 2 ). There was a similar improvement in the functional status of both patients ≥80 and <80 years. This was measured by a similar increase in the ejection fraction and similar improvement in the New York Heart Association class at 90-day follow-up. The 90-day composite of MAE rates between the patients ≥80 and <80 years were similar (45.6% vs 44.1%, p = 0.823) ( Table 4 ). No differences in MACCE 1 or MACCE 2 rates were observed between the 2 groups. Rates of vascular complications requiring surgery were low and comparable between patients ≥80 and <80 years. Fewer repeat revascularization events were observed in the ≥80-year-old group (p = 0.032) ( Table 4 ). The echocardiographic core laboratory analysis showed no evidence of aortic or mitral valve injury, chordal rupture, papillary muscle rupture, aortic aneurysm, or structural damage to heart chambers or septum in either groups. The time to MAEs curves to 90 days are depicted for both groups in Figure 1 . After adjusting for baseline, procedural, and angiographic characteristic differences identified in the univariate analysis, the multivariate logistic regression analysis confirmed that age was not a predictor of outcomes at 90 days.
| MAE (to 90 Days) | Patients With Age <80 Yrs (n = 368) | Patients With Age ≥80 Yrs (n = 59) | p Value |
|---|---|---|---|
| Composite MAE | 45.6% | 44.1% | 0.823 |
| Composite MACCE 1 | 31.1% | 30.5% | 0.922 |
| Composite MACCE 2 | 26.8% | 23.7% | 0.622 |
| Death | 10.7% | 8.5% | 0.610 |
| Myocardial infarction | 16.7% | 22.0% | 0.313 |
| Q-wave MI | 0.5% | 1.7% | 0.328 |
| Non-Q wave MI | 16.1% | 20.3% | 0.420 |
| CPK-MB ≥ 3× ULN | 16.1% | 20.3% | 0.420 |
| CPK-MB ≥ 8× ULN | 10.4% | 13.6% | 0.466 |
| Stroke/TIA | 1.6% | 3.4% | 0.359 |
| Repeat revascularization | 10.4% | 1.7% | 0.032 |
| Need for cardiac or vascular operation ∗ | 3.3% | 1.7% | 0.512 |
| Acute renal dysfunction | 10.1% | 13.6% | 0.424 |
| Severe hypotension requiring treatment | 11.2% | 11.9% | 0.881 |
| Cardiopulmonary resuscitation/ventricular arrhythmia † | 11.7% | 8.5% | 0.461 |
| Aortic valve damage/increase in aortic insufficiency | 0.0% | 0.0% | — |
| Angiographic failure | 3.0% | 1.7% | 0.573 |
∗ Cardiac, thoracic or abdominal operation, or vascular operation for limb ischemia.
Demographic, baseline, and procedural characteristics were similar between patients who received percutaneous left ventricular support with the Impella 2.5 compared with patients who received the IABP in ≥80-year-old patients but with a few exceptions: (a) patients ≥80 years randomized to receive the Impella 2.5 more frequently had severe lesion calcification compared with patients randomized to the IABP (40.3% vs 20.3%, p = 0.008) and (b) patients randomized to the Impella 2.5 had a lower prevalence of American College of Cardiology/American Heart Association class A lesions as compared with the IABP group (4.2% vs 13.5%, p = 0.047) ( Table 5 ).
| <80 Year Old Population | ≥80 Years Old Population | |||||
|---|---|---|---|---|---|---|
| Impella 2.5 (n = 186) | IABP (n = 182) | p Value | Impella 2.5 (n = 30) | IABP (n = 29) | p Value | |
| Age | 64.8 ± 9.3 | 64.4 ± 9.4 | 0.645 | 84.1 ± 3.2 | 82.9 ± 2.8 | 0.129 |
| Gender – male | 82.3% | 81.9% | 0.922 | 70.0% | 82.8% | 0.249 |
| Prior myocardial infarction | 70.3% | 66.5% | 0.435 | 63.3% | 65.5% | 0.861 |
| CHF | 90.3% | 81.9% | 0.019 | 96.7% | 89.7% | 0.284 |
| PCI | 42.2% | 40.3% | 0.722 | 33.3% | 24.1% | 0.436 |
| CABG | 41.9% | 29.1% | 0.010 | 23.3% | 27.6% | 0.708 |
| Peripheral vascular disease | 25.1% | 28.6% | 0.459 | 26.7% | 17.2% | 0.383 |
| Diabetes mellitus | 55.9% | 50.0% | 0.256 | 36.7% | 44.8% | 0.524 |
| Renal insufficiency | 22.0% | 25.4% | 0.448 | 26.7% | 58.6% | 0.013 |
| History of tobacco use | 77.0% | 70.6% | 0.159 | 37.9% | 57.1% | 0.146 |
| Current tobacco user | 55.3% | 42.5% | 0.036 | 0.0% | 12.5% | 0.223 |
| LVEF | 23.26 ± 6 | 23.84 ± 6 | 0.379 | 23.97 ± 7.2 | 25.41 ± 6 | 0.422 |
| Additive EuroScore | 8.18 ± 3 | 7.77 ± 3 | 0.234 | 13.97 ± 13 | 12.34 ± 3 | 0.514 |
| STS mortality score | 4.86 ± 5 | 5.20 ± 7 | 0.571 | 11.50 ± 8 | 12.10 ± 8 | 0.773 |
| Number of lesions treated | 2.88 ± 1.46 | 2.86 ± 1.47 | 0.901 | 2.83 ± 1.3 | 2.93 ± 1.6 | 0.802 |
| Number of stents placed | 3.13 ± 1.82 | 2.85 ± 1.83 | 0.153 | 2.80 ± 1.5 | 3.17 ± 2.0 | 0.418 |
| Total lesion length | 37.30 ± 28 | 35.56 ± 26 | 0.535 | 30.1 ± 21.4 | 32.6 ± 27.6 | 0.695 |
| Use of RA during index procedure | 12.4% | 8.8% | 0.265 | 30.0% | 13.8% | 0.133 |
| Total RA # of passes | 6.15 ± 4.02 | 3.69 ± 3.6 | 0.013 | 6.4 ± 3.2 | 4.0 ± 3.5 | 0.166 |
| RA run time/lesion | 65.47 ± 42 | 49.32 ± 48 | 0.035 | 98.3 ± 58.8 | 17.5 ± 3.6 | 0.053 |
| Contrast use during index procedure | 275 ± 146 | 240 ± 116 | 0.012 | 222 ± 105.5 | 246 ± 112.8 | 0.405 |
| Severe calcification | 14.8% | 14.7% | 0.718 | 40.3% | 20.3% | 0.008 |
| Modified ACC/AHA lesion class | ||||||
| A | 8.2% | 5.3% | 0.072 | 4.2% | 13.5% | 0.047 |
| B1 | 21.1% | 19.3% | 0.494 | 15.3% | 18.9% | 0.559 |
| B2 | 40.2% | 41.8% | 0.623 | 55.6% | 43.2% | 0.137 |
| C | 30.5% | 33.7% | 0.300 | 25.0% | 24.3% | 0.925 |
| Total occlusion | 7.6% | 6.3% | 0.449 | 2.8% | 1.4% | 0.544 |
| Abrupt closure | 1.5% | 0.9% | 0.396 | 2.8% | 0.0% | 0.149 |
| Coronary perforation | 0.9% | 0.4% | 0.439 | 1.4% | 0.0% | 0.309 |
| Distal embolus | 0.0% | 0.7% | 0.077 | 4.2% | 0.0% | 0.076 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


