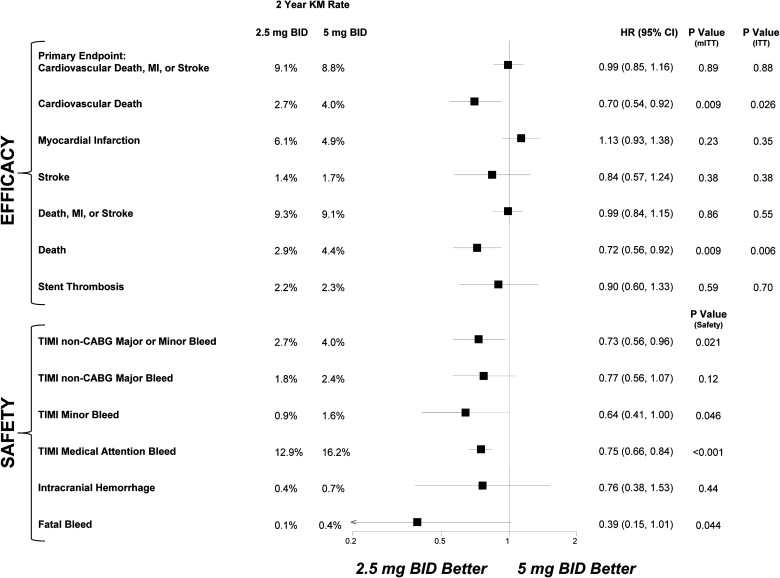
The dosing of anticoagulants is critical when balancing efficacy and safety. The Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With/Without Thienopyridine Therapy in Subjects With Acute Coronary Syndrome 2–Thrombolysis In Myocardial Infarction 51 (ATLAS ACS 2–TIMI 51) trial was designed to evaluate 2 low doses of rivaroxaban compared with placebo in patients with recent acute coronary syndromes being treated with antiplatelet therapies. Because the 2 doses significantly reduced the primary efficacy end point, a further comparison of the 2 treatment strategies was deemed important. In total, 15,526 patients were randomized to twice-daily rivaroxaban 2.5 mg, rivaroxaban 5 mg, or placebo. Comparing the 2 active doses, there were no significant differences between 2.5 and 5 mg for the primary efficacy end point of cardiovascular death, myocardial infarction, or stroke (9.1% vs 8.8%, p = 0.89), myocardial infarction (6.1% vs 4.9%, p = 0.23), or stent thrombosis (2.2% vs 2.3%, p = 0.59). However, there was a divergence in cardiovascular death, which included ischemic and hemorrhagic events, with the 2.5-mg dose resulting in lower rates than the 5-mg dose (2.7% vs 4.0%, p = 0.009). Notably, with 2.5 versus 5 mg, there were fewer study drug discontinuations (p = 0.004) and fewer non–coronary artery bypass grafting TIMI major or minor bleeds (p = 0.021) and fatal bleeds (p = 0.044). Of the patients who died, 8 in the 2.5-mg group and 20 in the 5-mg group experienced non–coronary artery bypass grafting TIMI major or minor bleeding events before death. In conclusion, the 2 doses of rivaroxaban reduced cardiovascular events in patients with recent acute coronary syndromes treated with antiplatelet therapies; however, the 2.5-mg dose was associated with lower mortality and fewer bleeding complications than the 5-mg dose. Thus, the addition of rivaroxaban 2.5 mg twice daily offers a more favorable balance of efficacy and safety in patients with recent acute coronary syndromes.
The factor Xa inhibitor rivaroxaban has been tested widely in patients with atrial fibrillation, pulmonary embolus, and venous thromboembolism. The underlying disease state and background antiplatelet therapies, however, have proved to be key determinants when dosing antithrombotic medications. As such, the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With/Without Thienopyridine Therapy in Subjects With Acute Coronary Syndrome–Thrombolysis In Myocardial Infarction 46 (ATLAS ACS–TIMI 46) trial was designed as a large, phase 2, dose-finding trial to test a broad range of rivaroxaban doses (total daily doses of 5 to 20 mg) in once-daily and twice-daily regimens in 3,491 patients with recent acute coronary syndromes (ACS) treated with antiplatelet therapies. Although there was a dose-dependent increased risk in bleeding, interestingly the numerically largest reductions in cardiovascular events were observed with the lowest rivaroxaban doses when administered twice daily. On the basis of these observations, a phase 3 trial of 15,526 patients was designed to evaluate 2 low doses of rivaroxaban, 2.5 mg twice daily and 5 mg twice daily, as adjunctive therapy in post-ACS patients (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With/Without Thienopyridine Therapy in Subjects With Acute Coronary Syndrome 2–Thrombolysis In Myocardial Infarction 51 [ATLAS ACS 2–TIMI 51]). As previously published, these rivaroxaban doses, compared with placebo, reduced significantly the primary efficacy end point of cardiovascular death, myocardial infarction (MI), or stroke. The present analysis provides a direct comparison of rivaroxaban 2.5 mg twice daily and 5 mg twice daily. The analysis presents information pertaining to the findings seen with the 2 treatment groups, including evaluation of cardiac events; the relation between bleeding and subsequent death; discontinuation of medical therapies; and subgroup analyses comparing the factors that may be related to the different pharmacokinetic profiles. Evaluating the low (5 mg twice daily) and very low (2.5 mg twice daily) doses of rivaroxaban adds to our understanding of the properties of factor Xa inhibition.
Methods
ATLAS ACS 2–TIMI 51 was a randomized, double-blind, placebo-controlled, event-driven trial. The study included patients (aged ≥18 years, without an upper age limit) with symptoms suggestive of ACS and diagnoses of ST-segment elevation MI, non–ST-segment elevation MI, or unstable angina. Patients aged <55 years were required to have either diabetes mellitus or previous MIs in addition to the index ACS events.
Key exclusion criteria were a platelet count <90,000/μL, hemoglobin <10 g/dl, and creatinine clearance <30 ml/min at screening; clinically significant gastrointestinal bleeding <12 months before randomization; previous intracranial hemorrhage; and previous ischemic stroke or transient ischemic attack in patients who were taking aspirin and a thienopyridine.
Enrollment occurred 1 to 7 days after hospital admission for ACS. Patients needed to be stabilized before enrollment, with the initial management strategies (e.g., revascularization) completed. They received standard care, including low-dose aspirin therapy and a thienopyridine (either clopidogrel or ticlopidine per national or local guidelines). Patients were randomly assigned in a 1:1:1 fashion to twice-daily administration of rivaroxaban 2.5 mg, rivaroxaban 5 mg, or placebo and were followed for efficacy and safety events.
The primary efficacy end point was a composite of cardiovascular death, MI, or stroke (ischemic, hemorrhagic, or stroke of uncertain cause). Stent thrombosis was defined using Academic Research Consortium definitions. Safety end points were defined using the TIMI bleeding scale, and complete definitions of the efficacy and safety end points have been published. All components of the key efficacy and safety end points were adjudicated by a clinical events committee blinded to treatment group.
Testing was to occur between pooled doses of rivaroxaban and placebo at α = 0.05, on the basis of the log-rank test, stratified by the intention to use a thienopyridine. If this achieved statistical significance in favor of rivaroxaban, then each individual rivaroxaban dose was to be tested against placebo using a similar stratified log-rank test on the basis of the closed testing procedure. As prespecified, the 2 rivaroxaban doses were to be compared for the primary efficacy end point and key secondary end points. A total of 983 primary efficacy end point events provided approximately 96% power to detect a 22.5% relative reduction between pooled doses of rivaroxaban and placebo with a 2-sided α value of 0.05. Although the comparison of the 2 rivaroxaban doses was prespecified, specific power calculations for this analysis were not conducted.
The active treatment groups were compared using hazard ratios and 2-sided 95% confidence intervals. As done previously, testing was conducted on the basis of the stratified log-rank test. Rates of the end points were expressed as Kaplan-Meier estimates through 24 months. Results were examined by relevant subgroups, and interaction testing was performed. The efficacy and safety populations have been previously described, with data for the efficacy end points corresponding to the modified intention-to-treat analysis set with a sensitivity analysis provided for the intention-to-treat analysis set ( online supplement , Figure 1 ). Data for the safety end points correspond to the safety analysis set.
Results
The baseline characteristics for the 2.5- and 5-mg twice-daily treatment groups were similar ( Supplementary Table 1 ). The mean duration of treatment with the study drug was 13.2 months with rivaroxaban 2.5 mg twice daily and 12.9 months with rivaroxaban 5 mg twice daily (p = 0.030). In subjects who received ≥1 dose of study drug, 26.9% of those administered rivaroxaban 2.5 mg twice daily and 29.4% of those administered rivaroxaban 5 mg twice daily prematurely discontinued the study drug (p = 0.004); the rate in the placebo arm was 26.4%. In total, 92.1% and 92.5% of patients in the 2.5- and 5-mg twice-daily groups were treated with clopidogrel, and in patients receiving dual-antiplatelet therapy, the mean duration of treatment with a thienopyridine was 13.3 months with rivaroxaban 2.5 mg twice daily and 13.1 months with rivaroxaban 5 mg twice daily (p = 0.29).
Comparing the 2 active treatment arms, there was no significant difference between 2.5 and 5 mg twice daily of rivaroxaban when assessing the primary end point (p = 0.89; Figure 1 ). Additionally, there were no statistically significant differences in the rates of MI (p = 0.23) or stent thrombosis (p = 0.59) between the 2 doses. When considering cardiac events broadly, including nonfatal MI, fatal MI, and fatal complications potentially related to ischemic heart disease (deaths that were sudden or not witnessed or deaths that were due to congestive heart failure, cardiogenic shock, or arrhythmia), there was again no difference in the 2 doses. Specifically, in the rivaroxaban 2.5- and 5-mg twice-daily treatment groups, the total numbers of these cardiac events were 268 and 256 (p = 0.68), respectively; each was significantly lower than in the placebo group (p = 0.017 and p = 0.005; Table 1 ).
| Event Type | Rivaroxaban | Placebo | ||||
|---|---|---|---|---|---|---|
| 2.5 mg Twice Daily | 5 mg Twice Daily | |||||
| Nonfatal MI | 186 | 144 | 202 | |||
| Fatal MI | 18 | 30 | 23 | |||
| Other fatal complications potentially related to ischemic heart disease | 64 | 82 | 103 | |||
| Fatal sudden or unwitnessed event | 55 | 59 | 81 | |||
| Fatal congestive heart failure/cardiogenic shock | 8 | 19 | 17 | |||
| Fatal arrhythmic event | 1 | 4 | 5 | |||
| Total: nonfatal MI + fatal MI + other fatal complications related to ischemic heart disease | 268 ∗,‡ | 256 ∗,† | 328 †,‡ | |||
∗ p = 0.68, 2.5 mg twice daily versus 5 mg twice daily.
† p = 0.005, 5 mg twice daily versus placebo.
However, in the 2.5-mg twice-daily group, 18 of 204 (8.8%) of the new MIs were fatal compared with 30 of 174 (17.2%) in the 5-mg twice-daily group (p = 0.074; Table 1 ). The fraction of overall cardiac events that were fatal differed between the 2 groups, with 82 of the 268 recurrent events (30.6%) being fatal in the 2.5-mg twice-daily group compared with 112 (43.8%) of the 256 events in the 5-mg twice-daily group. This difference was significant (p = 0.025). Likewise, there was a divergence between the 2.5 mg and 5 mg twice daily doses with regard to the incidence in the overall category of cardiovascular death (p = 0.009; Figure 2 ). Notably, cardiovascular death, by definition, included ischemic as well as hemorrhagic events (e.g., intracranial hemorrhage and gastrointestinal bleeds). Total mortality also was significantly lower with 2.5 versus 5 mg twice daily (p = 0.009; Figure 1 ).




