Conduction disorders and permanent pacemaker implantation are common complications in patients who undergo transcatheter aortic valve implantation (TAVI). The aim of this study was to assess the incidence and clinical significance of new bundle branch block in patients who underwent TAVI with the Medtronic CoreValve Revalving System (MCRS) or the Edwards SAPIEN valve (ESV). Data from 238 patients with no previous pacemaker implantation, left bundle branch block (LBBB) or right bundle branch block at baseline electrocardiography who underwent TAVI with either MCRS (n = 87) or ESV (n = 151) bioprostheses from 2007 to 2011 were analyzed. New-onset LBBB occurred in 26.5% patients (n = 63): 13.5% with the ESV (n = 20) and 50.0% with the MCRS (n = 43) (p = 0.001). Permanent pacemaker implantation was required in 12.7% of patients (n = 8) because of complete atrioventricular block (ESV n = 2, MCRS n = 4), LBBB and first degree atrioventricular block (MCRS n = 1) and new-onset LBBB associated with sinus bradycardia (MCRS n = 1). At discharge, LBBB persisted in 8.6% of ESV patients (n = 13) and 32.2% of MCRS patients (n = 28) (p = 0.001). On multivariate analysis, the only predictor of LBBB was MCRS use (odds ratio 7.2, 95% confidence interval 2.9 to 17.4, p <0.001). Persistent new-onset LBBB at discharge was not associated with overall (log-rank p = 0.42) or cardiovascular (log-rank p = 0.46) mortality. New-onset right bundle branch block was documented in 4.6% of patients (n = 11), with no statistically significant differences between the ESV and MCRS. In conclusion, new-onset LBBB is a frequent intraventricular conduction disturbance after TAVI with a higher incidence with the MCRS compared with the ESV. LBBB persists in most patients, but in this cohort, it was not a predictor of overall or cardiovascular mortality or permanent pacemaker implantation.
The development of new left bundle branch block (LBBB) is associated with higher rates of complete atrioventricular block (AVB), syncope, and sudden cardiac arrest in the long term. New-onset LBBB after surgical aortic valve replacement is more common than complete AVB, and its incidence has recently been reported to be as high as 18%. Transcatheter aortic valve implantation (TAVI) has been developed for high-risk patients with severe symptomatic aortic valve stenosis who have been refused conventional surgical aortic valve replacement. After TAVI, the incidence of new LBBB has been reported to vary from 29% to 65% after implantation of the Medtronic CoreValve Revalving System (MCRS; Medtronic, Inc., Minneapolis, Minnesota) and from 6% to 18% after implantation of the Edwards SAPIEN valve (ESV; Edwards Lifesciences, Irvine, California). The pathophysiology of new conduction disturbances is probably related to the very superficial location of the left bundle branch in the uppermost part of the leftward ventricular septum and its close proximity to the aortic valve complex. A number of studies indicate that patient- and procedure-related factors such as septal wall thickness, noncoronary cusp thickness, depth of valve implantation within the left ventricular outflow tract, postimplantation bioprosthesis expansion, and the type of bioprosthesis play a role. The aims of the present study were (1) to evaluate the incidence of bundle branch block, (2) to investigate the need for permanent pacemaker implantation (PPI) after LBBB, (3) to identify the clinical, anatomic, and procedural predictors of new-onset LBBB, and (4) to assess the clinical outcomes of patients who developed new LBBB.
Methods
From November 2007 to November 2011, a total of 366 patients underwent TAVI at our center using the balloon-expandable ESV (Cribier-Edwards, Edwards SAPIEN, or SAPIEN-XT; Edwards Lifesciences) or the self-expandable MCRS. Of these, 128 patients were excluded because of previous pacemaker implantation, cardiac resynchronization therapy, and/or implantable cardioverter-defibrillator implantation (n = 38); LBBB (n = 41); or right bundle branch block (RBBB) (n = 49) on baseline electrocardiography. The final TAVI study population therefore consisted of 238 patients. All patients underwent standard 12-lead electrocardiography before the procedure. To assess intraoperative conduction disturbances, 7-lead continuous electrocardiographic monitoring was performed. After the procedure, continuous electrocardiographic monitoring was routinely performed in all patients during the hospital stay. Postoperatively, 12-lead electrocardiography was performed daily during hospitalization to detect any alterations in atrioventricular and intraventricular conduction. The presence of conduction disturbances at any time was defined by the presence of ≥1 of the following abnormalities: first-, second-, or third-degree AVB, LBBB, RBBB, left anterior hemiblock, or left posterior hemiblock. The currently accepted criteria were used to code each of these conduction disturbances. The requirement for PPI was determined by the attending cardiologist according to the standardized criteria of the American College of Cardiology, American Heart Association, and Heart Rhythm Society. Annular diameter measurements were averaged from computed tomographic and transthoracic or transesophageal echocardiographic data, with annular dimensions measured at the level of the leaflet hinges as described by Roman et al. The preoperative valve orifice area was obtained echocardiographically by using the continuity equation. Standard transthoracic echocardiography was performed in all patients before and after TAVI to assess left ventricular function (ejection fraction), aortic stenosis morphology and severity (area in square centimeters and mean and peak gradients in millimeters of mercury), as well as to verify bioprosthesis position and function after implantation. Standard views were obtained. Valve morphology assessment, valve area planimetry, and ascending aortic and aortic annular dimensions were routinely measured as part of the examination. Pre- and postdilatation of the valve, the depth of bioprosthesis implantation, and the ratio between bioprosthesis size and the aortic annulus were evaluated. The implantation height of the final prostheses placement was measured in 149 patients using fluoroscopic aortography in a left oblique projection that displayed the aortic valve in optimal alignment, with all 3 leaflets visible en face. The depth of delivery was defined as the distance from the native aortic annular margin on the side of the right coronary cusp (leftward on the described projection) and on the side of the left coronary cusp (rightward on the described projection) to the most proximal edge (deepest in the left ventricle) of the deployed bioprosthesis stent frame. The ratio between the bioprosthesis nominal diameter and native annular size was calculated as the prosthesis/annulus ratio.
Qualitative variables are expressed as percentages and quantitative variables as mean ± SD or median (interquartile range) depending on variable distribution. Comparisons of numeric variables were performed using the 2-sided Student’s t test and the chi-square or Fisher’s exact test was used to compare qualitative variables. A stepwise logistic regression analysis including all variables with p values <0.20 on univariate analysis was used to determine the independent predictors of LBBB. Statistical analysis was performed using SPSS version 13.0 for Windows (SPSS, Inc., Chicago, Illinois). Two-sided p values <0.05 were considered as indicators of statistical significance.
Results
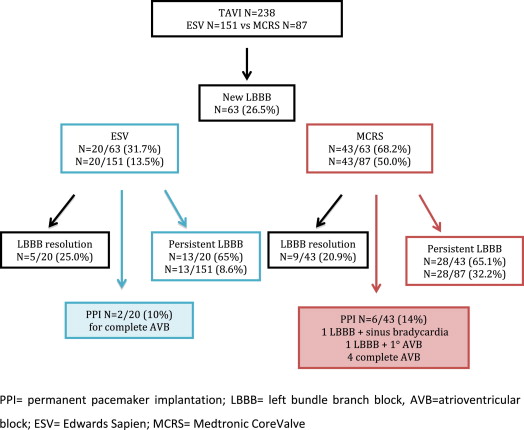
The clinical, echocardiographic, and preprocedural data of 238 TAVI patients without previous PPI, LBBB, or RBBB on baseline electrocardiography are listed in Table 1 . There were no relevant differences between the MCRS and the ESV cohorts, except for a higher percentage of men in the ESV group (59.6% vs 43.7%, p = 0.013). Ten patients (4.2%) underwent valve-in-valve implantation for degenerated surgical aortic bioprostheses: 4 patients (1.7%) had prosthetic valve stenosis and 6 patients (2.5%) had mixed stenosis and regurgitation. A total of 203 patients (85.3%) underwent the procedure for predominant aortic stenosis, 3 patients (1.3%) for severe aortic regurgitation, and 32 patients (13.4%) for mixed aortic disease. The mean calculated logistic European System for Cardiac Operative Risk Evaluation score of the study population was 22.4 ± 15.1, and 154 patients (64.7%) were in New York Heart Association functional class III or IV. Implantation success was achieved in 232 of the 238 patients (97.5%). The main access site was transfemoral (84.0%); ESVs were implanted in 134 patients (67%) and MCRS prostheses in 66 patients (33%). The other access sites were transapical (5.8%), transaxillary (9.6%), and transaortic (0.42%). In the transaortic and transapical approaches, only ESVs were used, and in the transaxillary approach, MCRS prostheses were implanted in 91.3%. Balloon predilatation was used in 200 patients (84.0%). Postdilatation was performed in a total of 45 patients (10 with ESVs vs 35 with MCRS prostheses, p = 0.001). The maximum depth of the base of the valve below the aortic annulus was measured on postprocedural angiography and averaged 6.4 ± 3.3 mm at the right border and 5.9 ± 3.1 mm at the left border, and it was deeper in the MCRS group (right border 8.5 ± 3.8 vs 5.0 ± 1.9 mm, p = 0.001; left border 7.9 ± 3.5 vs 4.6 ± 1.8 mm, p = 0.001). The ratio between bioprosthesis valve size and the annulus was higher in the MCRS than the ESV group (1.16 ± 0.06 vs 1.07 ± 0.07 p = 0.001). To evaluate the incidence and nature of postinterventional arrhythmias, we analyzed the pre- and postprocedural and discharge electrocardiograms of our patients. Sinus rhythm was detected in 197 patients (82.7%) before TAVI and in 187 patients (78.6%) after TAVI. The mean preprocedural PR interval was 178.3 ± 39.3 ms. For QRS duration, there was a significant increase from 97.6 ± 13.5 ms before TAVI to 113.4 ± 32.9 ms after TAVI, with an increase of 12.8 ± 27.5 ms (p <0.001). After the procedure, new-onset LBBB ( Figure 1 ) was documented in 63 patients (26.5%), 20 (13.5%) with ESVs and 43 (50.0%) with MCRS prostheses (p = 0.001). Of these, 15 (6.3%) had first AVB in association, 2 with ESVs and 13 with MCRS prostheses (p = 0.001). During the hospital stay, 8 patients (12.7%) with new-onset LBBB underwent PPI because of complete AVB in 6 (ESV n = 2, MCRS n = 4), LBBB plus first AVB in 1 (MCRS), and new-onset LBBB associated with sinus bradycardia in 1 (MCRS). The median time from TAVI to PPI was 4 days (interquartile range 2 to 5). In 14 patients, LBBB was temporary, with restoration of the normal intraventricular conduction pattern at discharge (5 ESV vs 9 MCRS patients). LBBB persisted in 13 ESV patients (8.6%) and 28 MCRS patients (32.2%) (p = 0.001) at discharge. New-onset RBBB was documented in 11 patients (4.6%) (5 with ESVs and 6 with MCRS prostheses, p = 0.20), 1.7% in association with left anterior hemiblock, 0.4% with first-degree AVB, and 0.8% with left anterior hemiblock and first-degree AVB. There were no statistically significant differences between ESV and MCRS bioprostheses ( Table 2 ).
| Characteristic | TAVI Overall (n = 238) | ESV (n = 151) | MCRS (n = 87) | p Value |
|---|---|---|---|---|
| Age (yrs) | 79.4 ± 7.6 | 79.7 ± 8.4 | 78.9 ± 6.1 | 0.40 |
| Men | 128 (53.8%) | 90 (59.6%) | 38 (43.7%) | 0.013 |
| Body mass index (kg/m 2 ) | 26.2 ± 4.75 | 26.3 ± 4.8 | 26.02 ± 4.6 | 0.59 |
| Previous valve | 10 (4.2%) | 10 (6.6%) | 0 (0.0%) | 0.015 |
| Coronary artery disease | 93 (39.1%) | 55 (36.4%) | 38 (43.7%) | 0.21 |
| Hypertension | 179 (75.2%) | 110 (72.8%) | 69 (79.3%) | 0.28 |
| Diabetes mellitus | 65 (27.3%) | 39 (25.8%) | 26 (29.9%) | 0.54 |
| New York Heart Association class III or IV | 154 (64.7%) | 100 (66.2%) | 54 (62.1%) | 0.57 |
| Glomerular filtration rate <60 ml/min | 132 (55.5%) | 82 (54.7%) | 50 (57.5%) | 0.55 |
| Logistic European System for Cardiac Operative Risk Evaluation score | 22.4 ± 15.1 | 22.6 ± 15.9 | 22.05 ± 19.9 | 0.78 |
| Society of Thoracic Surgeons score | 8.4 ± 8.4 | 7.8 ± 8.03 | 9.4 ± 9.1 | 0.19 |
| Ejection fraction | 52.3 ± 12.2 | 54.2 ± 10.5 | 50.1 ± 13.5 | 0.02 |
| Ejection fraction <35% | 31 (13.2%) | 14 (9.3%) | 17 (19.5%) | 0.028 |
| Pulmonary hypertension >60 mm Hg | 31 (13.2%) | 18 (11.9%) | 13 (14.9%) | 0.54 |
| Annulus (mm) | 23.3 ± 1.8 | 23.0 ± 1.7 | 23.9 ± 1.8 | 0.001 |
| Valvular area (cm 2 ) | 0.89 ± 1.8 | 0.91 ± 2.2 | 0.84 ± 0.99 | 0.80 |
| Mean aortic gradient (mm Hg) | 54.8 ± 16.8 | 54.7 ± 15.9 | 54.9 ± 18.4 | 0.92 |
| Mitral regurgitation (moderate to severe) | 21 (8.8%) | 12 (7.9%) | 9 (10.3%) | 0.47 |
| Porcelain aorta | 41 (17.2%) | 24 (15.9%) | 17 (19.6%) | 0.48 |
| Conduction Abnormality | TAVI Overall (n = 238) | ESV (n = 151) | MCRS (n = 87) | p Value |
|---|---|---|---|---|
| LBBB | 63 (26.5%) | 20 (13.5%) | 43 (50%) | 0.001 |
| LBBB + AVB | 15 (6.3%) | 2 (1.3%) | 13 (14.9%) | 0.001 |
| RBBB | 11 (4.6%) | 5 (3.3%) | 6 (6.9%) | 0.20 |
| RBBB + AVB | 1 (0.4%) | — | 1 (1.1%) | 0.36 |
| RBBB + LAH | 4 (1.7%) | 1 (0.7%) | 3 (3.4%) | 0.10 |
| RBBB + LAH + AVB | 2 (0.8%) | 2 (1.3%) | — | 0.53 |
| First-degree AVB | 23 (9.7%) | 11 (7.3%) | 12 (13.8%) | 0.11 |
Table 3 lists the clinical, echocardiographic, and procedural variables of the 238 patients grouped according to new-onset LBBB after the procedure. Previous coronary artery bypass grafting, coronary artery disease, valve implantation depth into the left ventricular outflow tract, use of the MCRS bioprosthesis, and postdilatation were univariate predictors of new LBBB. On multivariate analysis, the only predictor of LBBB was MCRS use (odds ratio 7.2, 95% confidence interval 2.9 to 17.4, p <0.001). In 41 patients (17.2%), LBBB persisted at discharge. We observed no differences in term of New York Heart Association functional class at 1 year between the groups with and without persistent LBBB after discharge (class I 58.3% vs 62.8%, class II 25% vs 28.2%, class III 16.7% vs 6.4%, and class IV 0% vs 1.3%; p = 0.30). At 1-year follow-up, we observed 8 (20%) and 26 (15.4%) deaths in the groups with and without persistent new-onset LBBB at discharge, specifically, 4 (12.5%) and 12 (7.1%) cardiovascular deaths in patients with and without LBBB. The causes of cardiovascular death in patients with new-onset LBBB were sudden death (n = 2), pulmonary edema (n = 1), and cardiogenic shock (n = 1). LBBB was not a predictor of overall or cardiovascular mortality (log-rank p = 0.42 and 0.46) at a median of follow-up of 348.5 days (interquartile range 0 to 1,096), as shown in Figure 2 . In the group of patients with left ventricular ejection fractions <35%, 8 patients (12.7%) developed LBBB compared with 23 (13.1%) who did not (p = 0.54). Also, in this small group, LBBB was not a predictor of overall mortality at 1 year (log-rank p = 0.80). Also, new-onset LBBB was not a predictor of PPI (p = 0.73).
| Predictor | LBBB (n = 63) | No LBBB (n = 175) | p Value |
|---|---|---|---|
| Age (yrs) | 79.02 ± 6.7 | 79.6 ± 6.7 | 0.58 |
| Men | 30 (47.6%) | 98 (56.0%) | 0.39 |
| Body mass index (kg/m 2 ) | 25.6 ± 4.5 | 26.4 ± 4.8 | 0.56 |
| Coronary artery disease | 32 (50.8%) | 61 (34.8%) | 0.047 |
| Hypertension | 52 (82.5%) | 127 (72.6%) | 0.23 |
| Diabetes mellitus | 22 (34.9%) | 43 (24.6%) | 0.14 |
| New York Heart Association class III or IV | 42 (66.7%) | 112 (64.0%) | 0.75 |
| Estimated glomerular filtration rate <60 (ml/min) | 39 (61.9%) | 93 (53.2%) | 0.19 |
| Logistic European System for Cardiac Operative Risk Evaluation score | 22.7 ± 15.9 | 22.2 ± 14.8 | 0.8 |
| Society of Thoracic Surgeons score | 9.7 ± 11.4 | 7.5 ± 7.03 | 0.26 |
| Ejection fraction | 51.9 ± 12.3 | 52.9 ± 11.8 | 0.56 |
| Ejection fraction <35% | 8 (12.7%) | 23 (13.1%) | 0.54 |
| Mean aortic gradient (mm Hg) | 53.6 ± 13.6 | 55.4 ± 17.9 | 0.47 |
| Moderate to severe aortic regurgitation | 7 (11.1%) | 29 (16.6%) | 0.2 |
| Moderate to severe mitral regurgitation | 5 (7.9%) | 16 (9.1%) | 0.78 |
| Porcelain aorta | 11 (17.5%) | 30 (17.1%) | 1 |
| ESV | 20 (31.7%) | 128 (73.1%) | |
| MCRS | 43 (68.3%) | 43 (24.6%) | 0.0001 |
| Valve size (mm) | |||
| 23 | 9 (14.3%) | 64 (36.6%) | 0.001 |
| 26 | 27 (42.9%) | 85 (48.6%) | |
| 29 | 27 (42.9%) | 26 (14.9%) | |
| Valve implantation position | |||
| Right border (mm) | 8.4 ± 3.8 | 5.7 ± 2.8 | 0.001 |
| Left border (mm) | 7.8 ± 3.7 | 5.3 ± 2.6 | 0.001 |
| Predilatation | 53 (84.1%) | 147 (84.0%) | 1 |
| Postdilatation | 22 (34.9%) | 23 (13.1%) | 0.001 |
| Valve size/annulus ratio | 1.12 ± 0.1 | 1.10 ± 0.1 | 0.057 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


