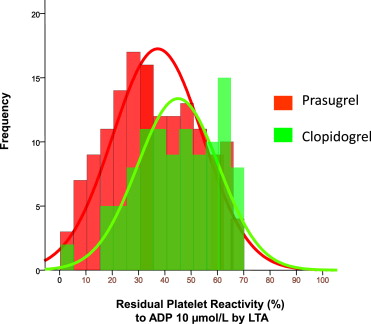
The everolimus-eluting stent (EES) performs better than the first generation drug-eluting stent. Prasugrel compared with clopidogrel in acute coronary syndromes treated invasively is associated with improved clinical outcome and decreased risk of stent thrombosis. The aim of the study was to compare the clinical outcome and degree of platelet aggregation inhibition of patients treated with EES for unprotected left main disease (ULMD) and receiving clopidogrel or prasugrel. Patients receiving an EES for ULMD and with low residual platelet reactivity on clopidogrel or prasugrel treatment were included in the analysis. The primary end point of the study was the composite of cardiac mortality and myocardial infarction at 1 year. The secondary end point was the degree of platelet aggregation inhibition as assessed by light transmittance aggregometry. From January 2009 to December 2011, 252 patients with low residual platelet reactivity on thienopyridine treatment were treated with EES for ULMD. Of these, 104 patients received clopidogrel and 148 received prasugrel. The primary end point rate was lower in the prasugrel group compared with clopidogrel group: 1.3% and 9.6%, respectively (p = 0.002). Residual platelet reactivity was less in the prasugrel group compared with clopidogrel group (adenosine diphosphate 10 μmol/L 37 ± 17% and 45 ± 15%, respectively, p <0.001). At multivariate analysis, prasugrel treatment was related to the primary end point (hazard ratio 0.17; 95% confidence interval 0.04 to 0.77, p = 0.022). In conclusion, in patients treated with EES for ULMD, prasugrel compared with clopidogrel is associated with increased platelet aggregation inhibition and a better clinical outcome.
Randomized trials and registries have shown that the everolimus-eluting stent (EES) performs better than the first generation drug-eluting stent. Prasugrel compared with clopidogrel in acute coronary syndromes treated invasively is associated with improved clinical outcome and decreased risk of stent thrombosis. No data exist on the comparison of prasugrel with clopidogrel in patients with unprotected left main disease (ULMD) treated with EES. The aim of this study was to compare the clinical outcome and the degree of platelet aggregation inhibition of patients treated with EES for ULMD and receiving clopidogrel or prasugrel.
Methods
The ULMD Florence registry started in 2004 and enrolled patients treated with drug-eluting stents for ULMD. Details on this registry have been previously published. From the registry, we identified patients who received exclusively EES (either XIENCE V; Abbott Vascular, Santa Clara, California or PROMUS; Boston Scientific Corp., Natick, Massachusetts) and those who had platelet reactivity assessment with adenosine diphosphate (ADP) 10 μmol/L by light transmittance aggregometry on clopidogrel or prasugrel treatment. The exclusion criteria from the study were (1) emergency percutaneous coronary intervention (PCI), (2) anticipated noncompliance to dual antiplatelet therapy for at least 1 year, and (3) high residual platelet reactivity on thienopyridine treatment (residual aggregation with ADP 10 μmol/L ≥70%). Patients underwent PCI instead of coronary surgery because of either patient’s preference or the high risk associated with surgery. High surgical risk was defined as a logistic EuroSCORE ≥6.
PCI was performed using standard techniques. For distal left main (LM) disease, a single-stent technique was preferred in patients with a normal or diminutive appearing side branch, whereas a double-stent technique was considered in patients with disease of both ostia and proximal segments of left anterior descending artery and circumflex artery. Whatever the stenting technique used, routine final kissing balloon after dilation with noncompliant balloons had to be performed in all cases.
Multivessel disease was defined as a stenosis of >70% of ≥1 major coronary arteries at baseline angiography besides the LM lesion. Diseases of left anterior descending artery and circumflex artery included lesions beyond 10 mm from the ostia. Completeness of revascularization was defined as the successful revascularization of all vessels with a diameter stenosis of >70% and a diameter >2 mm achieved either during the index hospitalization or at any time within 30 days after ULMD PCI. Procedural antithrombotic therapy included unfractionated heparin to achieve an activated clotting time of 200 to 250 seconds, whereas the use of glycoprotein IIb/IIIa inhibitors was at discretion of the operator.
All patients received a loading dose of clopidogrel (600 mg) or prasugrel (60 mg) before angiography. Chronic antiplatelet treatment included aspirin (300 mg/day indefinitely) and clopidogrel (75 mg/day) or prasugrel (10 mg/day for patients aged <75 years and 5 mg/day for patients aged ≥75 years) for at least 1 year. From April 2010, all patients with ULMD undergoing PCI were treated with prasugrel whatever be the thienopyridine loading they received before angiography.
Blood samples anticoagulated with 0.129 mol/L of sodium citrate (ratio 9:1) for platelet reactivity assessment was obtained at least after 12 hours from clopidogrel or prasugrel loading. For patients receiving in the catheterization laboratory, the loading dose of thienopyridine and glycoprotein IIb/IIIa inhibitor and for patients shifted to prasugrel therapy after clopidogrel loading, blood samples were obtained after 6 days while the patient was on maintenance dose of thienopyridine. Platelet-rich plasma, obtained by centrifuging whole blood for 10 minutes at 200 g , was stimulated with 10 μmo/L of ADP (Mascia Brunelli, Milan, Italy), and residual aggregation was assessed using an APACT 4 light transmittance aggregometer (Helena Laboratories, Milan, Italy). The 100% line was set using platelet-poor plasma and the 0 baseline established with platelet-rich plasma (adjusted from 18 × 10 9 /L up to 30 × 10 9 /L). Platelet aggregation (according to the Born’s method) was evaluated considering the maximal percentage of platelet aggregation in response to stimulus. High residual platelet reactivity was defined as platelet aggregation by ADP ≥70%.
All patients had scheduled examinations at 1, 6, and 12 months. All other possible information derived from hospital readmission or by the referring physician, relatives, or municipality live registries was entered into the prospective database. All eligible patients were scheduled for angiographic follow-up at 6 to 9 months. Unscheduled angiography was allowed on the basis of clinical indication.
The primary end point of the study was the composite of 1-year cardiac death and myocardial infarction (MI). Secondary end points were (1) cardiac death, (2) MI, (3) ischemic stroke, (4) stent thrombosis, (5) major bleeding, and (6) degree of platelet aggregation inhibition as assessed by light transmittance aggregometry. All deaths were considered cardiac unless an unequivocal noncardiac cause could be documented. The diagnosis of non–Q-wave MI was based on an increase of creatine kinase-MB isoenzyme or troponin I >3× the upper limit of normal or for patients with elevated values on admission, as a reelevation of creatine kinase-MB or troponin I values. A Q-wave MI was defined as the development of new Q waves in ≥2 electrocardiographic leads and in addition to creatine kinase-MB or troponin I elevation. Ischemic stroke was defined as an acute neurologic defect lasting >24 hours without computed tomographic evidence of bleeding. Stent thrombosis was defined according to the Academic Research Consortium criteria. Major bleeding was defined according to the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) criteria.
The study was approved by the Institutional Review Committee, and all patients gave informed written consent to intervention and the study.
Discrete data are summarized as frequencies, and continuous data are expressed as mean ± SD or median and interquartile range as appropriate. The chi-square test was used for comparison of categorical variables, and the unpaired 2-tailed Student t test or Mann-Whitney rank sum test was used to test differences among continuous variables. Survival curves were generated with the use of the Kaplan-Meier method, and the difference between groups was assessed by log-rank test. The multivariate analysis for the primary end point was performed by the forward stepwise Cox proportional hazards model. The following variables were tested: age (years), EuroSCORE, left ventricular ejection fraction <40%, intravascular ultrasound-guided PCI, completeness of revascularization, residual platelet reactivity (%), and prasugrel therapy. Propensity score analysis was performed using a logistic regression model from which the probability for the use of prasugrel was calculated for each patient. The variables entered into the model were age (years), male gender, diabetes, serum creatinine level >150 μmol/L, left ventricular ejection fraction <40%, EuroSCORE, LM stenting of both branches, and chronic total occlusion of right coronary artery. Model discrimination was assessed with the c statistic and goodness of fit with the Hosmer-Lemeshow test. Thereafter, a Cox multivariate analysis was performed to adjust the use of prasugrel therapy for propensity score used as continuous covariate.
A post hoc power analysis on primary end point was performed to exclude the possibility that the result of the study was because of chance. All tests were 2-sided, and a p value <0.05 was considered significant. Analyses were performed using the software package SPSS 19 (SPSS Inc., Chicago, Illinois).
Results
From January 2004 to December 2011, 610 patients were screened for ULMD PCI with drug-eluting stents. Of these, 252 patients underwent ULMD PCI receiving EES and had a residual platelet reactivity assessment with ADP 10 μmol/L <70% on thienopyridine treatment: 104 patients received clopidogrel treatment and 148 prasugrel ( Figure 1 ).
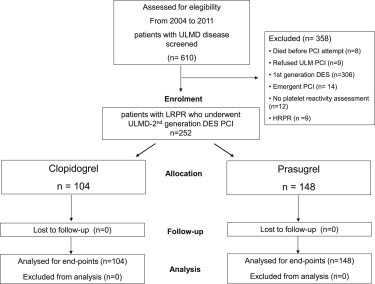
Table 1 summarizes the baseline clinical and angiographic characteristics. The 2 patient groups were similar in all baseline characteristics. Table 2 lists the procedural characteristics. Intravascular ultrasound-guided PCI was more frequent in the prasugrel group (84% vs 59%, p <0.001). The prasugrel group received more frequently stenting of both branches of LM, and as a consequence, the total LM stent length was higher compared with the clopidogrel group (25.3 ± 12.6 and 21.8 ± 10.4 mm, respectively, p = 0.024). Most patients in both groups had multivessel PCI and a complete coronary revascularization.
| Variable | Overall, n = 252 (%) | Clopidogrel, n = 104 (%) | Prasugrel, n = 148 (%) | p |
|---|---|---|---|---|
| Age (yrs) | 72 ± 11 | 72 ± 11 | 72 ± 10 | 0.811 |
| Men | 191 (76) | 78 (75) | 112 (76) | 0.902 |
| Current smokers | 36 (14) | 14 (13) | 22 (15) | 0.754 |
| Hypertension ∗ | 173 (68) | 73 (70) | 99 (67) | 0.579 |
| Diabetes mellitus | 59 (23) | 23 (22) | 36 (24) | 0.684 |
| Hypercholesterolemia † | 142 (56) | 60 (58) | 81 (55) | 0.641 |
| Peripheral vascular disease | 53 (21) | 20 (19) | 32 (22) | 0.644 |
| Previous MI | 56 (22) | 20 (19) | 36 (24) | 0.338 |
| Previous PCI | 102 (40) | 46 (44) | 56 (38) | 0.309 |
| Stable angina pectoris | 88 (35) | 43 (41) | 45 (30) | 0.073 |
| Acute coronary syndrome | 164 (65) | 61 (59) | 103 (70) | 0.073 |
| Creatinine >150 μmol/L | 32 (13) | 9 (9) | 23 (15) | 0.106 |
| LVEF (%) | 45 ± 12 | 45 ± 12 | 45 ± 12 | 0.923 |
| LVEF ≤40% | 86 (34) | 34 (33) | 51 (34) | 0.770 |
| EuroSCORE, median (IQR) | 6.9 (3.2–17.1) | 6.3 (3.1–12.3) | 7.9 (3.2–18.5) | 0.253 |
| EuroSCORE ≥6 | 139 (55) | 55 (53) | 84 (57) | 0.553 |
| LM plus 3-vessel disease | 89 (35) | 33 (32) | 56 (38) | 0.318 |
| Distal LM location | 234 (92) | 96 (92) | 137 (93) | 0.939 |
| Right coronary artery narrowing | 149 (59) | 60 (58) | 88 (60) | 0.779 |
| Right coronary artery total occlusion | 54 (21) | 24 (23) | 29 (20) | 0.504 |
∗ Defined as a history of hypertension treated with medication, diet, and/or exercise; blood pressure >140/90 mm Hg on ≥2 occasions or currently taking antihypertensive medication.
† History of a diagnosis and/or treatment of dyslipidemia by physician; criteria included documentation of total cholesterol level >200 mg/dl.
| Variable | Overall, n = 252 (%) | Clopidogrel, n = 104 (%) | Prasugrel, n = 148 (%) | p |
|---|---|---|---|---|
| Ostial/shaft LM stenting only | 17 (6) | 8 (8) | 9 (6) | 0.616 |
| Distal LM | 233 (92) | 96 (92) | 137 (93) | 0.939 |
| Single stent | 166 (66) | 73 (70) | 88 (59) | 0.081 |
| Stenting of both branches | 91 (36) | 31 (30) | 60 (40) | 0.081 |
| T stenting | 38 | 14 | 24 | |
| Crush stenting | 53 | 17 | 36 | |
| Total LM stent length (mm) | 23.8 ± 11.8 | 21.8 ± 10.4 | 25.3 ± 12.6 | 0.024 |
| IVUS guidance | 190 (75) | 66 (59) | 124 (84) | <0.001 |
| Abciximab | 110 (44) | 49 (47) | 61 (41) | 0.353 |
| IABP | 25 (10) | 8 (8) | 16 (11) | 0.406 |
| Maximum pressure inflation (atm) | 21 ± 2 | 22 ± 2 | 21 ± 2 | 0.204 |
| RVD before PCI (mm) | 3.77 ± 0.37 | 3.76 ± 0.41 | 3.77 ± 0.33 | 0.809 |
| MLD after PCI (mm) | 3.81 ± 0.36 | 3.82 ± 0.36 | 3.81 ± 0.36 | 0.833 |
| Multivessel PCI | 165 (65) | 65 (62) | 100 (68) | 0.405 |
| Complete revascularization | 206 (82) | 87 (84) | 119 (81) | 0.511 |
Residual platelet reactivity was lower in the prasugrel group compared with the clopidogrel group (37 ± 17% and 45 ± 15% respectively, p <0.001). Figure 2 depicts the distribution of residual platelet reactivity values in the 2 groups. The prasugrel group included 24 patients with high residual platelet reactivity on clopidogrel (ADP test 72 ± 5%). After shifting to prasugrel, all patients had low residual platelet reactivity, and the mean value of platelet aggregation at the ADP test was 41 ± 18%.