The present substudy from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial assessed the outcomes and their relation to different antithrombotic regimens in patients with previous coronary artery bypass grafting (CABG) treated with primary percutaneous coronary intervention. Of 3,599 patients with information regarding a history of CABG, 105 (2.9%) had previously undergone CABG. Of these 105 patients, 46 were randomized to heparin plus a glycoprotein IIb/IIIa inhibitor and 59 to bivalirudin. The patients with versus without previous CABG were less frequently triaged to primary percutaneous coronary intervention (83.8% vs 93.2%, p = 0.0002) and had a longer door-to-balloon time (median 1.9 vs 1.6 hours, p = 0.047), lower rates of final Thrombolysis In Myocardial Infarction flow grade 2 to 3 in the intervened vessel (92.6% vs 97.8%, p = 0.007), and less frequent rates of complete or partial ST-segment resolution (66.3% vs 77.6%, p = 0.019). At 3 years, previous CABG was associated with a significantly greater incidence of major adverse cardiovascular events (36.4% vs 21.4%, p <0.001) owing to greater rates of mortality (11.2% vs 6.7%, p = 0.08), reinfarction (11.6% vs 7.1%, p = 0.09), stroke (5.1% vs 1.8%, p = 0.013), and ischemic target vessel revascularization (23.6% vs 12.9%, p = 0.005). The outcomes did not differ significantly as a function of the antithrombotic regimen. On multivariate analysis, previous CABG was an independent predictor of 3-year ischemic stroke (hazard ratio 3.57, 95% confidence interval 1.09 to 11.66). Intervention on the saphenous vein graft versus the native vessel predicted 3-year major adverse cardiovascular events (hazard ratio 2.69, 95% confidence interval 1.17 to 6.19). In the HORIZONS-AMI trial, previous CABG was associated with a delay to mechanical reperfusion and lower rates of percutaneous coronary intervention and patency of the infarct related vessel along with worse clinical outcomes.
Limited information is available on the optimum treatment strategies in the setting of ST-segment elevation myocardial infarction (STEMI) and a history of coronary artery bypass grafting (CABG). In post hoc analyses from the randomized trials and registries of pharmacologic reperfusion in STEMI, previous CABG was associated with significantly greater mortality, and none of the fibrinolytic strategies was clearly advantageous in the improvement of outcomes. Similarly, when treated with mechanical reperfusion, the prognosis of patients with STEMI and previous CABG was remarkably worse compared to patients without a history of CABG. In the Second Primary Angioplasty in Myocardial Infarction Trial (PAMI-2), the 6-month mortality of patients with previous CABG treated with balloon angioplasty exceeded 3 times the death rate of patients without previous CABG. In the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial, which used stents in approximately 90% of the patients, the 90-day mortality was >2 times greater in patients with previous CABG. Our aim was to evaluate the clinical outcomes and their relation to different antithrombotic regimens in patients with previous CABG undergoing primary percutaneous coronary intervention (PCI) in the contemporary large-scale, multicenter, randomized Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial with the longest to date (3 years) follow-up duration.
Methods
The design and principal results of the HORIZONS-AMI trial have been previously reported. In brief, a total of 3,602 patients aged ≥18 years with acute MI within 12 hours of symptom onset and ST-segment elevation of ≥1 mm in ≥2 contiguous leads or new left bundle branch block or true posterior MI were randomized 1:1 to bivalirudin (Angiomax, The Medicines Company, Parsippany, New Jersey) alone or to unfractionated heparin plus a glycoprotein IIb/IIIa inhibitor. Immediate coronary angiography with left ventriculography was performed after randomization assignment, followed by triage to PCI, CABG, or medical management at the physician’s discretion. After patency was restored in the infarct-related vessel, eligible patients were randomly assigned again, in a 3:1 ratio, to either TAXUS Express2 paclitaxel-eluting stents or visually indistinguishable Express2 bare metal stents (both Boston Scientific, Natick, Massachusetts).
All electrocardiographic assessments were made at an independent electrocardiographic core laboratory that was unaware of the clinical and angiographic results. The absolute level of ST-segment elevation was measured with digital calipers to the nearest 0.01 mV, 20 ms after the end of the QRS interval, using the TP segment as the isoelectric baseline. The summed ST-segment resolution were categorized as none (<30%), partial (30% to 70%), and complete (>70%) at 60 minutes after the last contrast injection compared to the baseline electrocardiogram.
Information regarding whether previous CABG was performed was collected by the site investigators. An independent Clinical Events Committee who were unaware of the treatment assignment adjudicated all primary end points and stent thrombosis events using original source documents. The institutional review board at each center approved the present study. Each patient provided written informed consent before enrollment.
Two primary 30-day end points were included in the HORIZONS-AMI trial: major bleeding (not related to CABG) and the net adverse clinical events (major bleeding or major adverse cardiovascular events, including stroke, reinfarction, target vessel revascularization for ischemia, or death). Baseline anemia was defined using World Health Organization criteria. Renal insufficiency was defined as a creatinine clearance of <60 ml/min, calculated at baseline using the Cockcroft–Gault equation. Stent thrombosis was defined as definite or probable according to the Academic Research Consortium classification.
Categorical outcomes were compared using the chi-square test or Fisher’s exact test, and continuous variables using the Wilcoxon rank sum test. The primary event analyses were performed using time-to-event data (for which patients were censored at the point of withdrawal from the study or the last follow-up visit), are displayed using the Kaplan-Meier method, and were compared using the log-rank test. The 30-day and 3-year event rates are expressed as Kaplan-Meier estimates. Multivariate predictors of outcomes were defined using logistic regression analysis with stepwise selection using entry and exit criteria of p <0.1. The candidate variables entered into the model included age, gender, diabetes mellitus, chronic renal insufficiency, anemia, previous MI, a history of PCI, a history of CABG, door-to-balloon time, left ventricular ejection fraction <40%, saphenous vein graft (SVG) versus native coronary artery intervened vessel, stent implantation, use of an intra-aortic balloon pump, final Thrombolysis In Myocardial Infarction flow grade in the infarct-related vessel, and antithrombotic randomization.
Results
Of 3,602 patients with STEMI enrolled in the HORIZONS-AMI trial, information on a history of CABG was available for 3,599. Of these 3,599 patients, 105 (2.9%) had previously undergone CABG. The baseline clinical characteristics of the patients with and without previous CABG are listed in Table 1 . The patients with previous CABG were older, more frequently men, less frequently current smokers, had a greater prevalence of diabetes, hypertension, chronic renal insufficiency, anemia, previous MI, and previous PCI, and had ST-segment deviation to lesser extent on the baseline electrocardiogram. A lower proportion of patients with STEMI with previous CABG were triaged to primary PCI. Among the 17 patients with previous CABG not triaged to primary PCI, 14 were assigned to medical therapy (an inability to identify the culprit lesion and/or a lack of significant stenosis in 8, anatomy considered not amenable for revascularization in 3, a culprit vessel supplying a small amount of myocardium in 1, and unspecified reasons in 2 patients), 1 patient underwent deferred PCI, and 2 patients were referred for repeat CABG.
| Variable | Previous CABG | p Value | |
|---|---|---|---|
| Yes (n = 105) | No (n = 3,494) | ||
| Age (yrs) | <0.0001 | ||
| Median | 65 | 60 | |
| Interquartile range | 59–74 | 52–70 | |
| Men | 86% | 76% | 0.03 |
| Diabetes mellitus | 31% | 16% | <0.0001 |
| Hypertension | 80% | 53% | <0.0001 |
| Current smoking | 23% | 47% | <0.0001 |
| Chronic renal insufficiency | 33% | 16% | <0.0001 |
| Previous myocardial infarction | 60% | 9% | <0.0001 |
| Previous percutaneous coronary intervention | 49% | 10% | <0.0001 |
| Killip class ≥2 on admission | 4% | 9% | 0.08 |
| Baseline anemia ∗ | 19% | 10% | 0.006 |
| Leads with ST-segment elevation/depression (n) | <0.0001 | ||
| Median | 5 | 7 | |
| Interquartile range | 4–8 | 4–13 | |
| Maximum ST-segment elevation/depression (mm) | <0.0001 | ||
| Median | 2 | 3 | |
| Interquartile range | 2–3 | 2–4 | |
| Randomization to drug | |||
| Bivalirudin | 56% | 50% | 0.20 |
| Unfractionated heparin plus glycoprotein IIb/IIIa inhibitor | 44% | 50% | 0.20 |
| Primary management strategy | |||
| Primary percutaneous coronary intervention | 84% | 93% | 0.0002 |
| Deferred percutaneous coronary intervention | 1% | 0% | 0.06 |
| Coronary artery bypass grafting without percutaneous coronary intervention | 2% | 2% | 0.71 |
| Medical management | 13% | 5% | 0.0002 |
The baseline angiographic and procedural characteristics of the patients with and without previous CABG triaged to primary PCI (n = 88) are listed in Table 2 . In 56.8% of patients with previous CABG, primary PCI was performed on a native artery. Intervention on the left anterior descending artery or right coronary artery infarct vessels was less common, and the use of an aspiration catheter, thrombectomy, and/or a distal protection device was more common in patients with previous CABG. However, the rates of final Thrombolysis In Myocardial Infarction flow grade 0 or 1 were greater in the patients with previous CABG likely because of lower rates of successful stenting.
| Variable | Previous CABG | p Value | |
|---|---|---|---|
| Yes (n = 88) | No (n = 3,494) | ||
| Infarct-related coronary vessel | |||
| Left anterior descending | 11% | 33% | <0.0001 |
| Right coronary | 30% | 48% | 0.0005 |
| Left circumflex | 14% | 18% | 0.30 |
| Left main | 0% | 1% | 1.00 |
| Saphenous vein graft | 45% | 0% | <0.0001 |
| >1 Vessel treated | 7% | 4% | 0.15 |
| Use of aspiration catheter | 24% | 11% | 0.003 |
| Use of thrombectomy device | 5% | 1% | 0.01 |
| Use of distal protection device | 14% | 0.2% | <0.0001 |
| Use of intra-aortic balloon pump | 5% | 6% | 0.60 |
| Thrombolysis In Myocardial Infarction flow | |||
| Baseline | |||
| Grade 0 or 1 | 63% | 65% | 0.59 |
| Grade 2 | 17% | 16% | 0.78 |
| Grade 3 | 20% | 19% | 0.69 |
| Final | |||
| Grade 0 or 1 | 7% | 2% | 0.007 |
| Grade 2 | 6% | 6% | 0.91 |
| Grade 3 | 86% | 92% | 0.06 |
| Stent randomization | |||
| Paclitaxel-eluting stent | 78% | 75% | 0.57 |
| Bare metal stent | 22% | 25% | 0.57 |
| Successful stenting | 85% | 93% | 0.002 |
| Total stent length (mm) | 0.25 | ||
| Median | 24 | 24 | |
| Interquartile range | 18–52 | 20–36 | |
| Left ventricular ejection fraction (%) | 0.50 | ||
| Median | 50 | 50 | |
| Interquartile range | 40–60 | 43–60 | |
| Left ventricular ejection fraction <40% | 22% | 15% | 0.08 |
| Fluoroscopy time (min) | <0.0001 | ||
| Median | 19 | 11 | |
| Interquartile range | 13–27 | 8–17 | |
| Contrast volume (ml) | <0.0001 | ||
| Median | 280 | 225 | |
| Interquartile range | 218–357 | 180–290 | |
| Interval from symptom onset to hospital arrival (h) | 0.55 | ||
| Median | 2.2 | 2.2 | |
| Interquartile range | 1.2–3.3 | 1.3–4.0 | |
| Interval from emergency room to catheterization laboratory (h) | 0.001 | ||
| Median | 1.1 | 0.8 | |
| Interquartile range | 0.6–1.5 | 0.5–1.2 | |
| Interval from symptom onset to balloon time (h) | 0.47 | ||
| Median | 3.9 | 3.7 | |
| Interquartile range | 2.7–6.1 | 2.7–5.6 | |
| Door to balloon time (h) | 0.047 | ||
| Median | 1.9 | 1.6 | |
| Interquartile range | 1.4–2.5 | 1.2–2.2 | |
| Interval from first to last angiogram (h) | <0.0001 | ||
| Median | 0.9 | 0.7 | |
| Interquartile range | 0.6–1.2 | 0.5–0.9 | |
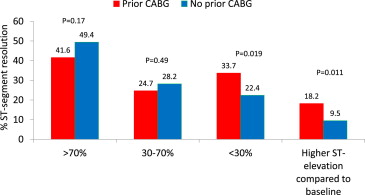
The patients with previous CABG had a lower mean percentage of ST-segment resolution at 60 minutes after their last angiogram compared to the baseline electrocardiogram (33.8 ± 92.5% vs 55.1 ± 60.6%, p = 0.048). They also had a significantly lower rate of complete or partial ST-segment resolution (66.3% vs 77.6%, p = 0.019) and a significantly greater rate of no ST-segment resolution and additional ST-segment elevation ( Figure 1 ).
An index PCI in patients with previous CABG was associated with a longer interval from the emergency room to the catheterization laboratory, a longer door-to-balloon time, and a longer procedure duration as assessed by the interval from the first to the last angiogram and by the fluoroscopy time. The volume of contrast medium was greater in patients with previous CABG, with a trend toward a greater incidence of increase in the serum creatinine level of ≥25% at 48 hours after the index procedure compared with the baseline value (21.4% vs 15.3%, p = 0.13).
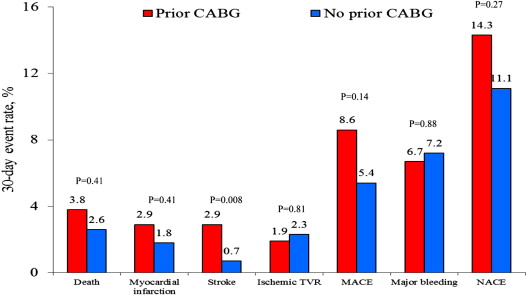
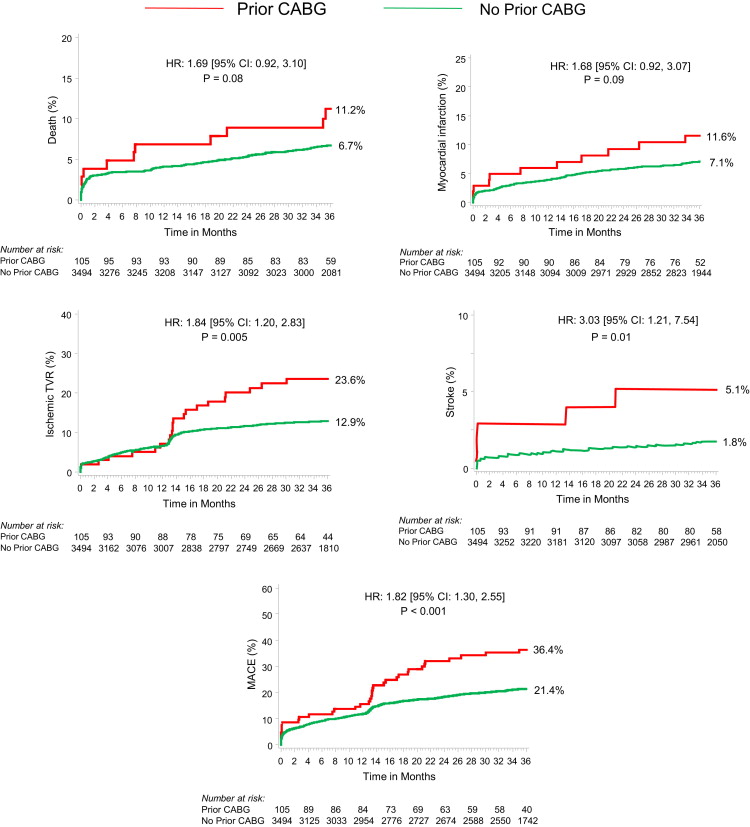
As shown in Figure 2 , the patients with and without previous CABG had no significant differences in the 30-day rates of major adverse cardiovascular events or net adverse clinical events. At 3 years of follow-up, however, previous CABG was associated with a significantly greater occurrence of major adverse cardiovascular events owing to greater rates of ischemic target vessel revascularization, with a trend toward an increased incidence of reinfarction and mortality ( Figure 3 ). Ischemic stroke occurred significantly more frequently in patients with previous CABG. A total of 5 patients developed ischemic stroke within 3 years (3 patients during the index hospitalization and 2 patients during the follow-up period). The occurrence of definite or probable stent thrombosis did not differ significantly in patients with and without previous CABG (1.3% vs 2.4% at 30 days, p = 0.52; and 2.6% vs 5.1 at 3 years, p = 0.36, respectively).
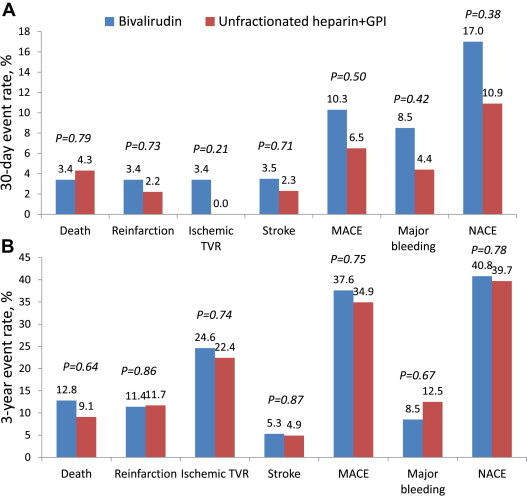
As shown in Figure 4 , in patients with previous CABG, the rates of major bleeding and major adverse cardiovascular events did not differ significantly among those randomized to bivalirudin (n = 59) or unfractionated heparin plus glycoprotein IIb/IIIa inhibitor (n = 46) at either early or late follow-up. The rate of definite or probable stent thrombosis was similar at 30 days (2.3% vs 0.0%, respectively, p = 0.37) and at 3 years (2.3% vs 3.0%, p = 0.87).
Of the 88 patients with previous CABG treated with primary PCI, intervention on the SVG or native vessel, or both, was performed in 33, 50, and 2 patients, respectively; information on the intervened vessel was missing for 3 patients. The main baseline characteristics of the patients with previous CABG undergoing intervention on the SVG or native vessel were well matched (data not shown). However, intervention on the SVG was associated with significantly less frequent achievement of final Thrombolysis In Myocardial Infarction flow grade 3 (73.7% vs 94.6%, p = 0.004), likely resulting in greater rates of a glycoprotein IIb/IIIa inhibitor administration after the procedure (0% vs 11.4%, p = 0.026).
The patients with an intervened SVG compared to patients with an intervened native vessel had significantly greater rates of major adverse cardiovascular events at 3 years, mainly from greater rates of mortality, reinfarction, and ischemic target vessel revascularization ( Table 3 ). However, no significant differences were found in the rates of major adverse cardiovascular events and its individual components among the patients with previous CABG and intervened native vessel compared to patients without previous CABG ( Table 3 and Figure 5 ).
| Variable | Patients With Previous CABG | p Value ∗ | Patients Without Previous CABG (n = 3,494) | p Value | ||
|---|---|---|---|---|---|---|
| SVG (n = 33) | Native (n = 50) | p 1 | p 2 | |||
| 30-day Outcomes | ||||||
| Net adverse clinical events | 15% | 14% | 0.93 | 11% | 0.46 | 0.45 |
| Major adverse cardiac events † | 12% | 4% | 0.17 | 5% | 0.08 | 0.69 |
| Death | 3% | 2% | 0.78 | 3% | 0.86 | 0.82 |
| Reinfarction | 3% | 2% | 0.77 | 2% | 0.59 | 0.91 |
| Death or reinfarction | 6% | 2% | 0.34 | 4% | 0.57 | 0.47 |
| Stroke | 3% | 2% | 0.77 | 0.7% | 0.10 | 0.24 |
| Ischemic target vessel revascularization | 3% | 0% | 0.22 | 2% | 0.17 | 0.28 |
| Stent thrombosis | 3% | 0% | 0.21 | 2% | 0.77 | 0.28 |
| Major bleeding | 3% | 10% | 0.24 | 7% | 0.36 | 0.40 |
| 3-yr Outcomes | ||||||
| Net adverse clinical events | 55% | 36% | 0.14 | 26% | 0.0002 | 0.13 |
| Major adverse cardiac events | 52% | 30% | 0.04 | 21% | <0.0001 | 0.19 |
| Death | 12% | 5% | 0.18 | 7% | 0.19 | 0.51 |
| Reinfarction | 20% | 9% | 0.17 | 7% | 0.006 | 0.70 |
| Death or reinfarction | 28% | 11% | 0.04 | 13% | 0.008 | 0.65 |
| Stroke | 3% | 4% | 0.82 | 2% | 0.52 | 0.19 |
| Ischemic target vessel revascularization | 36% | 22% | 0.18 | 13% | 0.0002 | 0.09 |
| Stent thrombosis | 7% | 0% | 0.07 | 5% | 0.61 | 0.12 |
| Major bleeding | 7% | 12% | 0.36 | 9% | 0.62 | 0.33 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


