There are few data available on the prognosis of painless ST-segment elevation myocardial infarction (STEMI). The aim of this study was to determine the incidence, clinical characteristics, and outcomes of painless STEMI. We analyzed the Korea Acute Myocardial Infarction Registry (KAMIR) study, which enrolled 7,288 patients with STEMI (61.8 ± 12.8 years old, 74% men; painless STEMI group, n = 763; painful STEMI group, n = 6,525). End points were in-hospital mortality and 1-year major adverse cardiac events (MACEs). Patients with painless STEMI were older and more likely to be women, nonsmokers, diabetic, and normolipidemic and to have a higher Killip class. The painless group had more in-hospital deaths (5.9% vs 3.6%, p = 0.026) and 1-year MACEs (26% vs 19%, p = 0.002). In Cox proportional hazards analysis, hypotension (hazard ratio [HR] 4.40, 95% confidence interval [CI] 1.41 to 13.78, p = 0.011), low left ventricular ejection fraction (HR 3.12, 95% CI 1.21 to 8.07, p = 0.019), and a high Killip class (HR 3.48, 95% CI 1.19 to 10.22, p = 0.023) were independent predictors of 1-year MACEs in patients with painless STEMI. In conclusion, painless STEMI was associated with more adverse outcomes than painful STEMI and late detection may have contributed significantly to total ischemic burden. These results warrant more investigations for methodologic development in the diagnosis of silent ischemia and painless STEMI.
Nonfatal ST-segment elevation myocardial infarction (STEMI) including painless STEMI can be unrecognized by the patient and discovered only on subsequent routine electrocardiographic examinations or at autopsy examination. Painless STEMI is often followed by silent myocardial ischemia. Silent myocardial ischemia is defined as an objective documentation of myocardial ischemia in the absence of angina pectoris or angina equivalents. Approximately 2.5% of myocardial ischemia in the study population have occurred in the absence of chest pain in several studies. In a cohort of 1,092 patients undergoing preoperative dobutamine stress echocardiography and noncardiac vascular surgery, unrecognized MI and silent myocardial ischemia were highly prevalent (23% and 28%). Silent myocardial ischemia seems to be an independent predictor of future cardiac morbidity and mortality. Also, it is estimated that the first manifestation of coronary artery disease in 60% to 70% of patients is sudden death or MI, whereas only a minority present first with angina pectoris or other symptoms of reversible myocardial ischemia. Nevertheless, the incidence of painless STEMI is unclear, and currently a paucity of data is available on the outcomes of painless MI. Hence, we aimed to examine the incidence, clinical characteristics, and outcomes of painless STEMI.
Methods
The Korea Acute Myocardial Infarction Registry (KAMIR), launched in November 2005, is a Korean prospective multicenter data collection registry reflecting real-world treatment practices and outcomes in Asian patients diagnosed with acute infarction. The registry includes 50 community and teaching hospitals with facilities for primary percutaneous coronary intervention (PCI) and on-site cardiac surgery. It is the largest acute MI registry in Korea and, to the best of our knowledge, 1 of the largest in the world. From November 2005 to December 2007, 14,885 patients with acute MI were enrolled in the KAMIR. Data were collected by a trained study coordinator using a standardized case-report form and protocol. The study protocol was approved by the ethics committee at each participating institution.
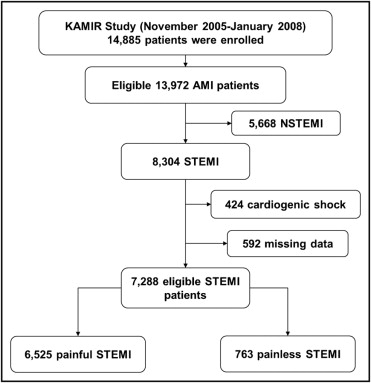
In the present study, patients with STEMI were selected (61.8 ± 12.8 years old, 74% men) and constituted the eligible 7,288 of the 14,885 total registered patients. Patients were grouped into the painless STEMI group (n = 763) and the painful STEMI group (n = 6,525) based on the presence of chest pain around the time of their visit to the emergency room ( Figure 1 ).
All patients received aspirin ≥100 mg and a loading dose of clopidogrel 300 to 600 mg and heparin. The maintenance dose was aspirin 100 mg/day and clopidogrel 75 mg/day. Aspirin and clopidogrel was administered to all patients for ≥6 months according to existing guidelines. Postintervention medication included aspirin, clopidogrel, β blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers. After discharge, the patients continued to receive the same medications that they received during hospitalization with the exception of some intravenous or temporary medications. Coronary interventions were performed using standard techniques. The decisions for predilation, direct stenting, postadjunctive balloon inflation, and the administration of glycoprotein IIb/IIIa receptor blockers were left to the discretion of individual operators. Clinical follow-up was performed at 1 month and 6, and 12 months and when anginalike symptoms occurred.
Acute MI was defined as the presence of ≥2 of the following 3 conditions: (1) ischemic symptoms, (2) increase of cardiac markers ≥2 times the upper limit of normal, or (3) new ST-segment elevation. STEMI was defined as a clinical presentation consistent with an acute MI and an electrocardiogram with ST-segment elevation ≥0.1 mV in ≥2 contiguous leads, Q wave, or new left bundle branch block. Painless STEMI was defined as STEMI without pain symptoms and dyspnea, which is an accepted angina equivalent.
Data on other cardiovascular risk factors such as hypertension, smoking, and previous ischemic heart disease were also reported by the patients themselves, with the exception of dyslipidemia, which was defined as a composite of self-reported history, previous statin usage, and a fasting cholesterol level ≥200 mg/dl.
Angiographic parameters such as Thrombolysis In Myocardial Infarction (TIMI) flow grade or American College of Cardiology/American Heart Association lesion type were assessed by the operator. A successful procedure was defined as <20% residual stenosis after the procedure in this study.
In-hospital deaths from all causes and 1-year major adverse cardiac events (MACEs) were considered primary outcomes. A MACE was defined as a composite of cardiac death, noncardiac death, nonfatal reinfarction, and coronary artery revascularization. Target vessel revascularization was defined as any repeated intervention driven by lesions in the treated vessel within and beyond the target lesion limits.
Continuous variables with normal distributions are presented as mean ± SD and were compared using Student’s t test or Mann–Whitney U test when group distributions were skewed. Categorical variables were compared using chi-square test or Fisher’s exact test, where appropriate. Cumulative mortality, death and nonfatal MIs, revascularization, and composite MACE rates were evaluated using the Kaplan–Meier method and compared using log-rank test. Cox regression analysis was performed to identify independent predictors of 1-year mortality and MACEs in painless STEMI. Variables with a p value <0.1 in univariate Cox analysis were tested. All statistical tests were 2-tailed and a p value <0.05 was considered statistically significant. All analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, Illinois).
Results
Baseline demographic characteristics are listed in Table 1 . Painless STEMI comprised 11% of total patients with STEMI. Patients in the painless group were older, were more likely to be women, and were more likely to have diabetes, but were less likely to have hyperlipidemia, to have a family history, and to be a smoker. The painless group had fewer previous MIs and no significant differences in left ventricular function were observed between the 2 groups. The painless group was less likely to be given primary PCI. The painless group had comparable blood pressure to the painful group but the painless group had faster heart rates. With regard to door-to-balloon time, the painless group was given primary PCI significantly later than the painful group. On electrocardiogram a Q wave was more frequently seen in the painless group than in the painful group. Laboratory testing indicated that renal function was worse in the painless group. There were no differences in high-sensitivity C-reactive protein level but the N-terminal pro–B-type natriuretic peptide level was much higher in the painless group. The lipid profile was better in the painless group: total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels were lower than those in the painful group.
| Variable | Painless (n = 763) | Painful (n = 6,525) | p Value |
|---|---|---|---|
| Age (years) | 65.2 ± 12.8 | 61.4 ± 12.8 | <0.001 |
| Men | 531 (70%) | 4,883 (75%) | 0.001 |
| Body mass index (kg/m 2 ) | 23.8 ± 8.1 | 24.1 ± 5.2 | 0.164 |
| Diabetes mellitus | 219 (29%) | 1,549 (24%) | 0.003 |
| Hypertension | 369 (49%) | 2,918 (45%) | 0.074 |
| Hyperlipidemia | 232 (33%) | 2,318 (39%) | 0.002 |
| Smoker | 438 (58%) | 4,041 (63%) | 0.009 |
| Family history of coronary disease | 33 (4.7%) | 451 (7.8%) | 0.004 |
| Previous myocardial infarction | 269 (36%) | 2,538 (39%) | 0.043 |
| Left ventricular ejection fraction (%) | 50.2 ± 13.0 | 50.6 ± 11.6 | 0.466 |
| Killip class III on presentation | 89 (12%) | 454 (7.2%) | <0.001 |
| Systolic blood pressure (mm Hg) | 127.8 ± 40.9 | 127.5 ± 31.3 | 0.798 |
| Heart rate (beats/min) | 80.4 ± 39.0 | 77.0 ± 26.3 | 0.022 |
| Primary percutaneous coronary intervention | 454 (61%) | 4,861 (77%) | <0.001 |
| Door-to-balloon time (minutes) | 133.5 ± 136.1 | 88.4 ± 94.8 | <0.001 |
| Q wave | 247 (32%) | 1,053 (16%) | <0.001 |
| Left bundle branch block | 9 (1.2%) | 39 (0.6%) | 0.060 |
| Glomerular filtration rate (ml/min) | 67.0 ± 36.1 | 75.7 ± 41.2 | <0.001 |
| Glucose (mg/dl) | 172.0 ± 87.0 | 171.3 ± 74.5 | 0.826 |
| High-sensitivity C-reactive protein (mg/dl) | 12.6 ± 72.5 | 14.9 ± 75.4 | 0.463 |
| Amino-terminal pro–B-type natriuretic peptide (pg/ml) | 3,755.3 ± 6,960.6 | 1,802.3 ± 4,575.8 | <0.001 |
| Maximum creatine kinase (IU/L) | 1,464.8 ± 2,345.9 | 1,813.1 ± 2,064.9 | <0.001 |
| Maximum creatine kinase-MB (IU/L) | 111.6 ± 185.7 | 197.7 ± 314.1 | <0.001 |
| Maximum cardiac troponin I (ng/ml) | 50.8 ± 77.6 | 65.9 ± 180.3 | 0.025 |
| Total cholesterol (mg/dl) | 177.5 ± 44.5 | 184.0 ± 43.9 | <0.001 |
| Triglycerides (mg/dl) | 117.4 ± 94.1 | 126.5 ± 113.2 | 0.017 |
| High-density lipoprotein cholesterol (mg/dl) | 45.1 ± 12.8 | 45.3 ± 22.7 | 0.819 |
| Low-density lipoprotein cholesterol (mg/dl) | 113.5 ± 39.2 | 118.4 ± 43.9 | 0.005 |
Coronary angiographic findings are presented in Table 2 . There were no significant differences between groups in culprit lesions. The painless group had more multivessel disease but less TIMI grade 0 flows before the procedure and complex lesions. Drug-eluting stents were used less frequently in the painless group and physicians used paclitaxel-eluting stents more in patients in the painless group than in those in the painful group.
| Variable | Painless (n = 763) | Painful (n = 6,525) | p Value |
|---|---|---|---|
| Culprit coronary lesion | |||
| Left main coronary artery | 12 (1.8%) | 78 (1.3%) | 0.259 |
| Left anterior descending coronary artery | 360 (54%) | 3,180 (52%) | 0.313 |
| Left circumflex coronary artery | 54 (8.1%) | 609 (9.9%) | 0.127 |
| Right coronary artery | 243 (36%) | 2,277 (37%) | 0.707 |
| Multivessel coronary disease | 395 (59%) | 3,239 (53%) | 0.003 |
| Thrombolysis In Myocardial Infarction grade flow | |||
| 0 (total occlusion) before procedure | 288 (45%) | 3,131 (53%) | <0.001 |
| 0 (no reflow) after procedure | 13 (1.7%) | 116 (1.8%) | 0.883 |
| 3 | 566 (71%) | 5,254 (92%) | 0.679 |
| Lesion complexity ⁎ | 446 (71%) | 4,486 (78%) | <0.001 |
| Stent profile | |||
| Use of drug-eluting stent | 504 (86%) | 4,986 (91%) | <0.001 |
| Sirolimus-eluting stent | 168 (29%) | 2,399 (44%) | <0.001 |
| Paclitaxel-eluting stent | 253 (43%) | 1,665 (31%) | <0.001 |
| Everolimus-eluting stent | 43 (7.4%) | 576 (11%) | 0.015 |
| Other drug-eluting stent | 40 (6.8%) | 346 (6.3%) | 0.642 |
| Use of bare metal stent | 81 (14%) | 469 (8.6%) | <0.001 |
| Stent diameter (mm) | 3.2 ± 0.4 | 3.2 ± 0.4 | 0.128 |
| Stent length (mm) | 24.9 ± 6.0 | 25.1 ± 6.3 | 0.326 |
⁎ Lesion types B2 to C according to the American College of Cardiology/American Heart Association.
Clinical outcomes in patients who underwent primary PCI are presented in Table 3 . The painless group had a larger number of in-hospital deaths. Other hospital outcomes including heart failure, acute renal failure, and bleeding tended to be worse and cardiogenic shock and stroke were significantly more frequent in the painless group. Long-term outcome analyses indicated that the composite MACE was greater in the painless group than in the painful group. In detail, all-cause death and any revascularization tended to be higher in percentages than in the painful STEMI group, although this was not statistically significant. In contrast, death, nonfatal MI, and target lesion revascularization rates were higher in the painless group. Kaplan–Meier analysis indicated that patients with painless STEMI had worse outcomes in death, MI, and composite MACE (p = 0.019 and 0.005, log-rank test, respectively). In contrast, all-cause death or revascularization rate did not show more than a trend to be worse in the painless group ( Figure 2 ). In multivariable Cox proportional hazard analysis, hypotension (hazard ratio [HR] 4.40, 95% confidence interval [CI] 1.41 to 13.78, p = 0.011), low left ventricular ejection fraction (HR 3.12, 95% CI 1.21 to 8.07, p = 0.019), and Killip class III (HR 3.48, 95% CI 1.19 to 10.22, p = 0.023) were independent predictors of 1-year MACEs in patients with painless STEMI. However, low left ventricular ejection fraction was not 1 of the predictors of 1-year mortality ( Figure 3 ).



