Previous data reported worse outcomes in female patients after acute ST elevation myocardial infarction (STEMI), related at least in part to less aggressive and nonparallel treatment. We investigated the presence of gender differences in left ventricular (LV) systolic and diastolic function in patients presenting with first STEMI, treated with primary percutaneous coronary intervention (PCI). Study population included 187 consecutive patients (81% men) presenting with STEMI and treated by primary PCI and guideline-based medications. Their mean age was 58 ± 10 years. All patients underwent a comprehensive echocardiographic evaluation within 3 days of admission. Female patients were older (62 ± 11 vs 59 ± 10 years, p = 0.006), with more co-morbidities and longer symptom duration (490 ± 436 vs 365 ± 437 minutes, p = 0.013). Echocardiography demonstrated that female patients had significantly lower LV systolic function (47 ± 8% vs 45 ± 8%, p = 0.03), lower septal and lateral e′ velocities, higher average E/e′ ratio (all p <0.001), elevated systolic pulmonary artery pressure (p = 0.03), and worse diastolic dysfunction (p = 0.007). No significant changes were present in left atrial volumes. In a logistic multivariate analysis model, female gender emerged as an independent predictor of septal e′ <8 cm/s (odds ratio 10.11, 95% confidence interval 1.23 to 82.32, p = 0.002) and E/average e′ ratio >15 (odds ratio 6.47, 95% confidence interval 1.63 to 25.61, p = 0.008). In conclusion, female patients undergoing primary PCI for first STEMI demonstrated worse systolic and diastolic LV function, despite receiving similar treatment as male patients.
Epidemiologic and clinical studies demonstrated that female patients have a worse survival after acute myocardial infarction (AMI). Some of the data indicate that the poor outcome is related to older age of women presenting with AMI, greater incidence of coronary artery disease risk factors and longer time to hospital admission after symptom onset; however, even after adjustment for age and other unfavorable prognostic factors, survival disadvantage was demonstrated in female patients with AMI. A possible explanation for these past findings included less aggressive treatment offered to female patients with AMI, especially primary percutaneous coronary intervention (PCI). Another possible explanation is that certain biologic differences between genders may contribute to an altered response to AMI. After AMI, left ventricular (LV) systolic and diastolic dysfunction are important prognostic factors; however, to date, no study evaluated gender-related differences in early LV function in patients with AMI treated with primary PCI. The aim of the present study was to investigate whether gender differences in LV function exist in patients with first acute ST elevation myocardial infarction (STEMI), all treated with primary PCI.
Methods
We performed a retrospective, single-center observational study. Included were 229 consecutive patients with an acute STEMI who had been admitted to the Cardiac Intensive Care Unit of the Tel-Aviv Sourasky Medical Center from May 2011 to December 2012. The baseline demographic, clinical, echocardiographic, and angiographic test results were retrieved from medical files and the hospital database. As previous myocardial damage can determinately affect cardiac function, we excluded 26 patients with previous myocardial infarction. Similarly, as acute hemodynamic instability may adversely affect preload and afterload, we excluded 7 other patients presenting with cardiogenic shock (defined as persistent systolic blood pressure <90 mm Hg and nonresponsive to fluid replacement or the need for inotropic agents or intra-aortic balloon pumping to maintain blood pressure ≥90 mm Hg). Nine additional patients were excluded because primary PCI was not performed (7 male and 2 female patients). The final study population included 187 patients, with 152 men (81%).
The diagnosis of STEMI was determined by a typical history of chest pain, diagnostic electrocardiographic changes, and serial elevation of serum cardiac biomarkers according to current guidelines. The electrocardiographic criterion for the diagnosis of STEMI was an ST-segment elevation of ≥1 mm in >2 adjacent leads or new left bundle branch block. Symptom duration was defined as the time from symptom onset (usually chest pain or discomfort) to emergency room admission. Door-to-balloon time was defined as the time elapsing from emergency room admission until balloon inflation in the culprit artery and blood flow restoration.
All patients received dual antiplatelet therapy consisting of aspirin (a loading dose of 300 mg followed by 100 mg/day) and thienopyridine (clopidogrel loading dose of 600 mg followed by 75 mg/day or prasugrel loading dose of 60 mg followed by 10 mg/day), as well as a heparin bolus (4,000 U) in the emergency department. Those who arrived to the hospital by mobile intensive care unit were administered aspirin and heparin while being transported. Primary PCI was performed within 90 minutes of hospital admission in patients with symptoms ≤12 hours in duration and in patients with symptoms lasting 12 to 24 hours if pain consisted at the time of admission. All patients were treated with heparin boluses during angioplasty aiming to obtain an activated clotting time of 200 to 250 seconds in patients treated with a IIb/IIIa antagonist and an activated clotting time of >250 seconds in patients not treated. The IIb/IIIa antagonist was administered during the primary PCI at the discretion of the senior operator. After admission to the Cardiac Intensive Care Unit, treatment with β blockers, statins, and renin and/or angiotensin blockers were started in all patients unless contraindicated.
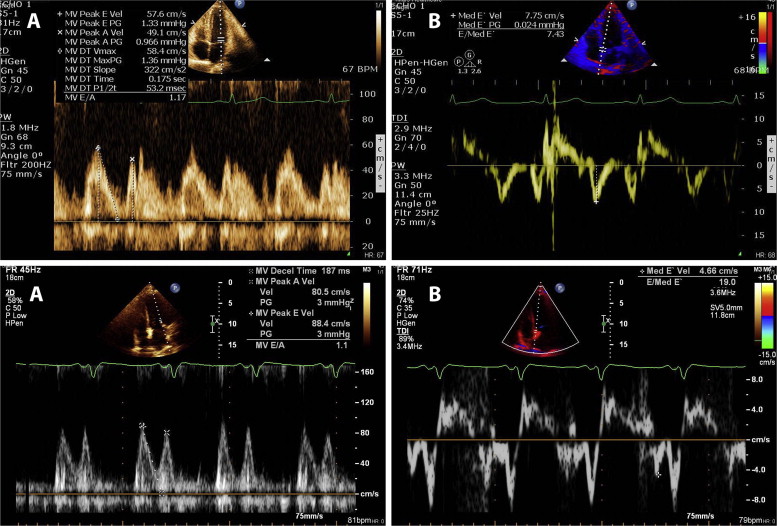
All patients underwent a screening echocardiographic examination within 3 days of admission. Echocardiography was performed using Philips iE33 equipped with S5-1 transducers (Philips Healthcare, Andover, Massachusetts) and GE Vivid 7 model equipped with M4S transducer. Echocardiograms were interpreted independently by 2 expert investigators. LV diameters and interventricular septal and posterior wall width were measured from the parasternal short axis by means of a 2-dimensional or a 2-dimensional–guided M-mode echocardiogram of the LV at the papillary muscle level using the parasternal short-axis view. LV ejection fraction was calculated by the biplane method. The 16-segment model was used for scoring the severity of segmental wall motion abnormalities according to the American Society of Echocardiography. Early transmitral flow velocity (E), late atrial contraction (A) velocity, and early diastolic mitral annular velocity (e′) were measured in the apical 4-chamber view to provide an estimate of LV diastolic function. The peak E/peak e′ ratio was calculated (mitral E/e′ ratio) from the average of at least 3 cardiac cycles, and the deceleration time of the E wave was also measured. Left atrial volume was calculated using the biplane area-length method at end-systole. Cardiac output was calculated as the product of LV outflow tract area, LV outflow tract flow integral, and heart rate as demonstrated on pulse-wave Doppler. Diastolic function was assessed by integrating measurements of the mitral inflow and Doppler tissue imaging of the mitral annulus using the medial annulus velocity and classified into 4 categories: normal diastolic function = 0, impaired relaxation = 1, pseudonormal = 2, and restrictive pattern = 3 based on recent guidelines.
Analysis was performed retrospectively using the institution’s electronic records and database, and the study protocol was approved by the institutional review board.
All data were summarized and are displayed as mean ± SD or median (25% to 75%) for continuous variables and as number (percentage) of patients in each group for categorical variables. The p values for the chi-square test were calculated with the Fisher’s exact test. Logistic regression models were performed, where LV ejection fraction <50%, septal e′ <8 cm/s, and E/average e′ >15 were defined as the dependent variables and adjusted to age, gender, hypertension, diabetes, anterior myocardial infarction location, and ischemia duration, defined as time from symptom onset to establishment of Thrombolysis In Myocardial Infarction 3 flow at the culprit artery during PCI. All of the analyses were considered significant at a 2-tailed p value of <0.05. The SPSS statistical package was used to perform all statistical evaluations (SSPS, Chicago, Illinois).
Results
Study population included 187 patients, mean age 58 ± 10 years (range 34 to 87), and 152 patients (81%) were male. Demographic, clinical, angiographic, and laboratory findings of the patient population are presented in Table 1 . Compared with male patients, female patients were older (p = 0.006), had significantly higher rate of dyslipidemia (p = 0.023), and presented later to hospital after symptom onset (490 ± 436 vs 365 ± 437 minutes, p = 0.013). However, there was no significant difference in door-to-balloon time and all other clinical and angiographic findings. No significant change was found in the extent of coronary artery disease, infarct location, creatinine level, peak creatine phosphokinase, troponin levels, and the occurrence of heart failure throughout hospitalization.
| Variable | Males (n = 152) | Females (n = 35) | p Value ∗ |
|---|---|---|---|
| Age (years) (mean ± SD) | 59 ± 10 | 62 ± 11 | 0.006 |
| Hypertension | 52 (34%) | 17 (49%) | 0.087 |
| Hyperlipidemia | 56 (37%) | 20 (57%) | 0.023 |
| Smoker | 95 (61%) | 17 (49%) | NS |
| Diabetes mellitus | 21 (14%) | 6 (17%) | NS |
| Anterior infarct location | 75 (49%) | 15 (43%) | NS |
| Symptom duration (minutes) (median ± SD) | 365 ± 437 | 490 ± 436 | 0.013 |
| Door to balloon time (minutes) (median ± SD) | 54 ± 13 | 53 ± 15 | NS |
| Number of narrowed coronary arteries: | |||
| 1 | 70 (46%) | 13 (38%) | NS |
| 2 | 53 (35%) | 11 (31%) | |
| 3 | 29 (19%) | 11 (31%) | |
| Proximal infarct-related artery narrowing | 80 (53%) | 20 (57%) | NS |
| Heart failure during hospitalization | 11 (7%) | 4 (11%) | NS |
| Peak Troponin (ng ± ml) (mean ± SD) | 48 ± 61 | 50 ± 61 | NS |
| Peak CPK (IU/l) (mean ± SD) | 1526 ± 1582 | 1353 ± 1572 | NS |
∗ The p values for the chi-square test were calculated by Fisher’s exact test.
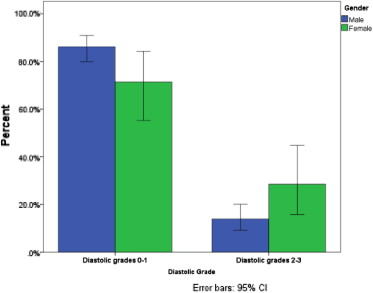
Median time to echocardiography was 1.4 days (interquartile range 1 to 2) for both groups. Table 2 presents the echocardiographic findings according to gender. Female patients had lower LV ejection fraction (44.8 ± 7.7 vs 47 ± 7.8, p = 0.003), lower e′ septal (5.68 ± 1.44 vs 6.96 ± 1.84 cm/s, p <0.001), e′ lateral (7.3 ± 2.05 vs 9.23 ± 2.64 cm/s, p = 0.001), and higher E/average e′ ratio (12.54 ± 4.6 vs 9.45 ± 2.6, p<0.001; Figure 1 ). In addition, systemic pulmonary artery pressure was higher in female patients (p = 0.03), as was an association with more advanced diastolic dysfunction grade (p = 0.007; Figure 2 ).
| Variable | Males (n = 152) | Females (n = 35) | p Value |
|---|---|---|---|
| Biplane LV ejection fraction (mean ± SD) | 47.0 ± 7.8 | 44.8 ± 7.7 | 0.03 |
| Wall motion index (mean ± SD) | 2.2 ± 0.35 | 1.7 ± 0.4 | NS |
| Heart rate (beats/min) (mean ± SD) | 75 ± 12 | 70 ± 12 | NS |
| Systolic blood pressure (mm/Hg) (mean ± SD) | 137 ± 19 | 134 ± 18 | NS |
| Diastolic blood pressure (mm/Hg) (mean ± SD) | 81 ± 13 | 80 ± 13 | NS |
| Cardiac output (L/min) (mean ± SD) | 5.2 ± 1.16 | 4.98 ± 1.27 | NS |
| Left atrial volume (ml 3 ) (mean ± SD) | 61.8 ± 16.4 | 57.2 ± 14.5 | NS |
| Left atrial volume index (ml/m 2 ) (mean ± SD) | 31.6 ± 8.0 | 32.4 ± 9.0 | NS |
| Mitral inflow E wave (cm/s) (mean ± SD) | 73.1 ± 17.2 | 76.7 ± 20.9 | NS |
| Mitral inflow E/A ratio (mean ± SD) | 1.16 ± 0.44 | 1.08 ± 0.49 | NS |
| Septal e’ (cm/s) (mean ± SD) | 6.96 ± 1.84 | 5.68 ± 1.44 | <0.001 |
| E wave velocity/septal e’ (mean ± SD) | 11.1 ± 3.8 | 14.3 ± 5.44 | <0.001 |
| Lateral e’ (cm/s) (mean ± SD) ∗ | 9.23 ± 2.64 | 7.3 ± 2.05 | 0.001 |
| E wave velocity/lateral e’ (mean ± SD) | 8.5 ± 2.68 | 11.3 ± 4.69 | 0.002 |
| E wave velocity/average e’ (mean ± SD) | 9.45 ± 2.6 | 12.54 ± 4.62 | <0.001 |
| Mitral E deceleration time (ms) (mean ± SD) | 179 ± 42 | 177 ± 48 | NS |
| LV end diastolic dimension (mm) (mean ± SD) | 49 ± 7 | 42 ± 16 | 0.002 |
| LV end systolic dimension (mm) (mean ± SD) | 31 ± 6 | 28 ± 12 | NS |
| Ventricular septal thickness (mm) (mean ± SD) | 12 ± 2 | 11 ± 2 | NS |
| Posterior LV wall thickness (mm) (mean ± SD) | 10 ± 2 | 9 ± 4 | 0.026 |
| Peak systolic PA pressure (mm/Hg) (mean ± SD) | 29 ± 4 | 33 ± 9 | 0.03 |
| Mitral regurgitation (any) | 35 (23%) | 13 (35%) | NS |
∗ Information on e’ lateral velocity was available in only 160 patients.