Pulmonary hypertension represents an important cause of morbidity and mortality in patients with mitral stenosis who undergo cardiac surgery, especially in the postoperative period. The aim of this study was to test the hypothesis that inhaled nitric oxide (iNO) would improve the hemodynamic effects and short-term clinical outcomes of patients with mitral stenosis and severe pulmonary hypertension who undergo cardiac surgery in a randomized, controlled study. Twenty-nine patients (4 men, 25 women; mean age 46 ± 2 years) were randomly allocated to receive iNO (n = 14) or oxygen (n = 15) for 48 hours immediately after surgery. Hemodynamic data, the use of vasoactive drugs, duration of stay, and short-term complications were assessed. No differences in baseline characteristics were observed between the groups. After 24 and 48 hours, patients receiving iNO had a significantly greater increase in cardiac index compared to patients receiving oxygen (p <0.0001). Pulmonary vascular resistance was also more significantly reduced in patients receiving iNO versus oxygen (−117 dyne/s/cm 5 , 95% confidence interval −34 to −200, vs 40 dyne/s/cm 5 , 95% confidence interval −34 to 100, p = 0.005) at 48 hours. Patients in the iNO group used fewer systemic vasoactive drugs (mean 2.1 ± 0.14 vs 2.6 ± 0.16, p = 0.046) and had a shorter intensive care unit stay (median 2 days, interquartile range 0.25, vs median 3 days, interquartile range 7, p = 0.02). In conclusion, iNO immediately after surgery in patients with mitral stenosis and severe pulmonary hypertension improves hemodynamics and may have short-term clinical benefits.
It has been shown that the pulmonary vasoconstriction present in clinical pulmonary hypertension can be alleviated in the short term using inhaled nitric oxide (iNO) through a mechanism involving vascular smooth muscle relaxation. In humans, the ability of iNO to reduce pulmonary vascular resistance (PVR) while sparing systemic resistance by selective pulmonary vasodilatation has been exploited in patients with right ventricular dysfunction, acute lung injury, and lung transplantation. Specifically in patients with mitral stenosis, in whom chronic left-sided underfilling is prominent, the use of iNO has not been studied thoroughly. Most studies were not randomized or controlled, did not look specifically at clinical outcomes but only focused on the immediate hemodynamic effects of iNO, used iNO during brief periods only, or were restricted to children or women. Fattouch et al compared the use of iNO in mitral stenosis with inhaled prostacyclin and intravenous nitroprusside but did not include a control group not using vasodilators. This is the first randomized controlled study of patients with severe mitral stenosis and pulmonary hypertension using iNO compared to a control group receiving only oxygen. We observed not only the hemodynamic effects of each treatment but also the associated short-term clinical outcomes in these patients.
Methods
The study was approved by the institutional research committee, and all subjects gave written informed consent. Patients were consecutively selected at an outpatient cardiac valve disease clinic of a tertiary cardiology referral hospital. Men and women aged ≥18 years were selected if they met all the following inclusion criteria: mitral stenosis with a valve area <1.5 cm 2 ; severe pulmonary hypertension, defined as pulmonary artery systolic pressure (PASP) >60 mm Hg; and symptomatic disease with New York Heart Association functional class ≥II. Patients were excluded if they presented with concomitant valvular disease other than mitral stenosis (specifically moderate or important mitral regurgitation as defined by preoperative quantitative echocardiography) or had severe left or right ventricular dysfunction, defined as an ejection fraction <40% by preoperative echocardiography. Nineteen screened patients were excluded before randomization because of concomitant mitral regurgitation (15 patients) or severe left ventricular dysfunction (3 patients). Central computerized randomization of the treatment assignments was performed. Concealment was interrupted only when the patient was weaned from cardiopulmonary bypass and started to receive the designated therapy.
Before surgery, baseline 2-dimensional echocardiography and Doppler echocardiography were performed using commercially available equipment (Sonos 5500; Philips Medical Systems, Andover, Massachusetts). The ejection fraction, transmitral valve gradient, valve area calculated using the pressure half-time method, mitral valve echocardiographic score, and PASP estimated using the modified Bernoulli equation were determined.
Before anesthesia induction in the operating room, a pulmonary artery catheter was placed in the right internal jugular vein or right subclavian vein, and pulmonary capillary wedge pressure, PASP, cardiac output calculated by thermodilution, and PVR were established. Cardiac output measurements were obtained from the mean of 3 consecutive end-expiratory readings. Patients were operated on using standard surgical procedures, and the choice of valve repair or replacement was left to the surgeons’ discretion.
Immediately before weaning off cardiopulmonary bypass, patients randomized to the iNO arm had the NO delivery equipment attached to the anesthesia breathing circuit. The surgical team was not aware of the patient randomization allocation until this moment. Inhaled NO was delivered using NO tanks connected to the inspiratory limb of the airflow tubes at a concentration of 10 ppm, a dose with the best cost/benefit ratio according to previous dosing studies. Concentrations of iNO and NO 2 were measured continuously with a dedicated monitoring device (NOxBox; Bedfont Instruments, Rochester, United Kingdom). Patients randomized to the oxygen control group continued to receive standard anesthesia care to maintain oxygen saturation >95%.
All patients were then transferred to a surgical intensive care unit (ICU) and continued the assigned treatment for up to 48 hours, in the ICU or in the ward. The criteria for ICU discharge were prespecified as hemodynamic stability (defined by a mean systemic arterial pressure >65 mm Hg), mechanical ventilation weaning with no signs of respiratory distress (as evaluated by the attending physician, who was blind to the outcomes of the study), urinary output >0.5 ml/kg/hour, and no significant bleeding from surgical sites. No patient was held in the ICU because of the protocol if he or she was considered apt for discharge and achieved the mentioned criteria. After extubation, patients received iNO or oxygen through a facial mask, keeping the aforementioned parameters. In all patients, an airtight sealed nonrebreathing cushioned face mask was used, allowing minimum leakage of either iNO or oxygen. Inhaled NO concentration was measured through a sampling line adjacent to the mask to guarantee a precise reading of the delivered concentration.
The use of any vasoactive drugs was permitted throughout the study and was left to the choice of the attending physician in the ICU, blinded to the outcomes of the study. At 24 and 48 hours after the initiation of iNO or control oxygen, a new reading of the pulmonary artery catheter was carried out, with the determination of the same parameters obtained before surgery. After 48 hours, patients withdrew oxygen therapy unless contraindicated by the assisting physician. Patients receiving iNO were progressively weaned, with total switch to room air or oxygen by mask as needed in 1 hour. All patients were followed during the total hospital stay for the assessment of predefined complications (acute renal insufficiency, infections, need for reintubation, liver failure, and death).
The primary outcomes of this study were the differences at 48 hours compared to the baseline cardiac index and PVR in each treatment group. These primary end points were chosen to allow a more accurate sample size calculation as well as to provide a more relevant link to the clinical effects. Secondary outcomes included clinical variables regarding postoperative complications, total days in the ICU, total hospital stay, and number and dosing of systemic intravenous vasoactive drugs. Complications were defined as acute renal insufficiency (renal output <0.3 ml/kg/hour), need for reintubation, sepsis according to standardized definitions, cardiogenic shock, and need for urgent reoperation.
Data are presented as mean ± SD, except for total hospital and ICU length of stay, which were not normally distributed and are presented as median and interquartile range. Comparisons among absolute values of the hemodynamic parameters preoperatively and at 24 and 48 hours were done using analysis of variance for repeated measures on ranks with Tukey’s and profile tests for time and group differences. Analyses of the differences as well as other comparisons regarding the iNO and control groups were made using unpaired Student’s t tests for continuous variables, Mann-Whitney or chi-square tests for proportions, and Fisher’s exact tests as needed. Sample size was calculated on the basis of an α error of 0.05 and power of 80% to detect a 40% difference in PVR between the 2 groups. We calculated the number of subjects needed for the study to be 30 (15 in each group) on the basis of previous studies. All calculations were done using intention-to-treat analysis and were performed in SAS version 9.1.3 (SAS Institute Inc., Cary, North Carolina). Significance was assumed at a 2-tailed p value <0.05.
Results
Twenty-nine patients (86% women, mean age 46 ± 2 years) were enrolled in the study, with a total evaluation of 48 patients. One patient withdrew consent on the morning of the surgery and was not included in the final analysis. The baseline clinical characteristics of the 2 groups are listed in Table 1 . No significant differences between the groups were observed. Patients enrolled were characterized by significant mitral stenosis (mean valve area 0.89 ± 0.04 cm 2 ) with severe pulmonary hypertension (mean PASP 73 ± 3 mm Hg, mean PVR 303 ± 31 dyne/s/cm 5 ). Only 1 death occurred; a patient in the oxygen group died 22 days after surgery with sepsis and multiple-organ failure. All randomized patients received the assigned treatment with iNO or oxygen during the full duration of the planned intervention, with no crossovers. Patients in the iNO group were mechanically ventilated for 7.5 ± 8.5 hours, while patients receiving oxygen were ventilated for 15.1 ± 21.8 hours (p = 0.23). No patient developed significant mitral valve regurgitation as assessed by echocardiography 7 days after surgery (data not shown).
| iNO | Oxygen | ||
|---|---|---|---|
| Variable | (n = 14) | (n = 15) | p Value |
| Age (years) | 48 ± 11 | 44 ± 13 | 0.43 |
| Women | 13 (93%) | 12 (80%) | 0.60 |
| Body mass index (kg/m 2 ) | 23 ± 4 | 22 ± 4 | 0.69 |
| New York Heart Association functional class | 0.39 | ||
| II | 3 (21%) | 6 (40%) | |
| III | 10 (72%) | 7 (47%) | |
| IV | 1 (7%) | 2 (13%) | |
| Atrial fibrillation | 8 (57%) | 8 (53%) | 0.99 |
| Left ventricular ejection fraction (%) | 71 ± 8 | 70 ± 6 | 0.48 |
| Mitral valve echocardiographic score | 10.1 ± 1.7 | 10.6 ± 1.0 | 0.55 |
| Mitral valve area (cm 2 ) | 0.92 ± 0.18 | 0.85 ± 0.21 | 0.35 |
| Mitral valve mean diastolic gradient (mm Hg) | 14.9 ± 3.7 | 16.4 ± 5.8 | 0.48 |
| PASP (echocardiography) (mm Hg) | 80 ± 21 | 80 ± 17 | 0.71 |
| Pulmonary capillary wedge pressure (mm Hg) | 26.3 ± 10.5 | 27.9 ± 10.5 | 0.87 |
| PASP (pulmonary artery catheterization) (mm Hg) | 73 ± 10 | 73 ± 14 | 0.99 |
| Cardiac index (L/min/m 2 ) | 2.35 ± 0.6 | 2.89 ± 0.9 | 0.10 |
| PVR (dyne/s/cm 5 ) | 341 ± 183 | 264 ± 133 | 0.38 |
| Bypass time (minutes) | 88 ± 31 | 94 ± 34 | 0.59 |
| Valve replacement | 6 (43%) | 3 (20%) | 0.25 |
| Reoperation | 4 (29%) | 3 (20%) | 0.91 |
| Intensive care unit stay (days) | 2.0 (0.25) | 3.0 (7.0) | 0.02 |
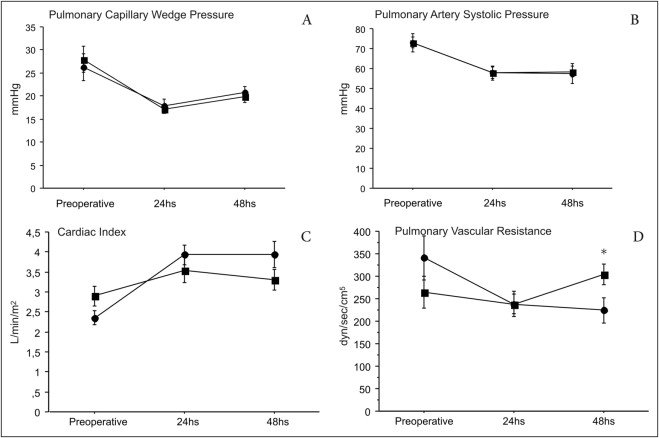
Changes in hemodynamic parameters after surgery are presented in Figures 1 and 2 . After 24 and 48 hours, the 2 groups had significant reductions in pulmonary capillary wedge pressure, with no differences between patients receiving iNO or oxygen. Significant decreases also occurred in PASP at 24 and 48 hours compared to that at baseline in the 2 groups, with no differences between them. However, the cardiac index increased significantly in patients receiving iNO at 24 and 48 hours (p <0.0001 for both). Moreover, although the cardiac index increased significantly compared to that at baseline in the 2 groups after 24 hours, this increase was sustained at 48 hours only in patients who received iNO, with a mean increase of 1.58 L/min/m 2 (95% confidence interval [CI] 1.0 to 2.16, p <0.0001) versus 0.4 L/min/m 2 (95% CI 0.01 to 0.82, p = 0.06) in patients receiving oxygen. PVR changes compared to those at baseline were also observed only in the group with iNO at 24 hours (−103 dyne/s/cm 5 , 95% CI −14 to −192, p = 0.04) and 48 hours (−117 dyne/s/cm 5 , 95% CI −34 to −200, p = 0.02), with significant differences between the 2 groups at 48 hours (p = 0.005). Partial pressure of oxygen was not significantly different between the groups at baseline or at 24 and 48 hours (baseline iNO group 92 ± 7 vs 93 ± 9 mm Hg in the oxygen group, p = 0.90; 24 hours 190 ± 72 vs 221 ± 97 mm Hg, p = 0.40; 48 hours 158 ± 67 vs 193 ± 102 mm Hg, p = 0.50). Although we did not invasively monitor the hemodynamic status of patients after weaning of iNO or oxygen, no patient in the study experienced any clinically relevant hemodynamic symptoms after withdrawal of either therapy.
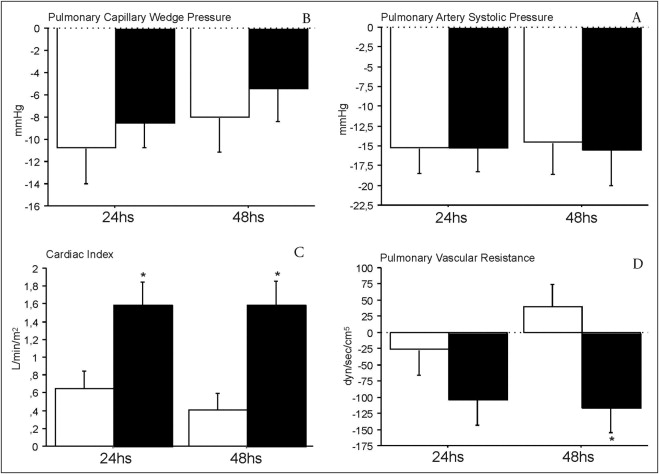
Patients who received iNO had significantly shorter ICU stays compared to patients who received oxygen ( Table 1 ). However, total hospital stay was similar in the 2 groups (median 10 days, interquartile range 6.0 in iNO group vs median 13 days, interquartile range 16.25 in the oxygen group, p = 0.14).
The use of concurrent vasoactive drugs might have interfered with the results regarding the previous hemodynamic measurements. Table 2 lists systemic intravenous vasoactive drugs used in the 2 groups during ICU and hospital stay. Although no significant differences were observed regarding the percentage of patients using each drug or the maximum dose used in each patient, the mean number of systemic vasoactive drugs used during hospital stay was significantly smaller in the iNO group (2.1 ± 0.14) compared to that in patients receiving only oxygen (2.6 ± 0.16) (p = 0.046). There were a nonsignificant smaller proportion of patients receiving nitroprusside and milrinone in the iNO group compared to controls. Other drugs known to significantly affect pulmonary pressure were not used by any patient in our study either during ICU or ward stay.



