Chronic right ventricular apical pacing (RVAP) has been associated with negative hemodynamic and clinical effects. The aim of the present study was to compare RVAP with right ventricular septal pacing (RVSP) in terms of echocardiographic features and clinical outcomes. A total of 93 patients without structural heart disease and with an indication for a permanent pacemaker were randomly assigned to receive a screw-in lead either in the RV apex (n = 46) or in the RV mid-septum (n = 47). The patients were divided into 3 subgroups according to the percentage of ventricular pacing: control group (n = 21, percentage of ventricular pacing ≤10%), RVAP group (n = 28), or RVSP group (n = 32; both latter groups had a percentage of ventricular pacing >10%). The RVAP group had more intraventricular dyssynchrony and a trend toward a worse left ventricular ejection fraction compared to the RVSP and control groups at 12 months of follow-up (maximal delay to peak systolic velocity between any of the 6 left ventricular basal segments was 57.8 ± 38.2, 35.5 ± 20.6, and 36.5 ± 17.8 ms for RVAP, RVSP, and control group, respectively; p = 0.006; mean left ventricular ejection fraction 62.9 ± 7.9%, 66.5 ± 7.2%, and 66.6 ± 7.2%, respectively, p = 0.14). Up to 48.1% of the RVAP patients showed significant intraventricular dyssynchrony compared to 19.4% of the RVSP patients and 23.8% of the controls (p = 0.04). However, no overt clinical benefits from RVSP were found. In conclusion, RVAP was associated with increased dyssynchrony compared to the RVSP and control patients. RVSP could represent an alternative pacing site in selected patients to reduce the harmful effects of traditional RVAP.
Although RVAP is known to be associated with asynchronous ventricular activation, at present, limited echocardiographic data are available regarding the effects of alternative pacing sites on the ventricular activation pattern. The aim of the present study was to prospectively compare the echocardiographic features and clinical outcomes of patients undergoing right ventricular apical pacing (RVAP) or right ventricular septal pacing (RVSP).
Methods
We conducted a randomized, single-center, single-blind, prospective study in which patients with an indication for permanent cardiac pacing because of atrioventricular block or sick sinus syndrome were randomly assigned to receive an active fixation lead either in the right ventricular apex or in the right ventricular mid-septum.
To further characterize the effect of different pacing sites on the echocardiographic and clinical/biologic features, the patients were divided into subgroups according to the percentage of ventricular pacing (%VP) obtained at the baseline evaluation (during the first week after device implantation). Thus, the patients who had had minimal pacing (%VP ≤10%) constituted the control group, and those with %VP >10% constituted the study group and were divided into 2 subgroups, depending on the location of the ventricular pacing lead according to randomization (RVAP and RVSP groups). The 10% cutoff value was selected with the thought that this percentage could best divide the subset of patients with minimal pacing (mainly those with sick sinus syndrome or paroxysmal atrioventricular block) from the rest of the population sample, who were expected to require ventricular pacing most of the time (permanent atrioventricular block). We also took into account that in the Mode Selection Trial (MOST), the lowest risk of heart failure hospitalization was observed in patients with a mean %VP of ≤10%. All patients with sick sinus syndrome or paroxysmal atrioventricular block received a pacemaker with true mode change algorithms, which were thus programmed in the AAI ↔ DDD mode to prevent unnecessary right ventricular pacing.
Patients were excluded before randomization if they met any of the following criteria: age <18 years; the presence of heart failure or any significant structural heart disease (left ventricular [LV] hypertrophy >15 mm, LV ejection fraction [EF] <50%, moderate or greater degrees of valvulopathy, previous myocardial infarction, or significant coronary artery disease); chronic pulmonary heart disease, and any musculoskeletal disease hampering the realization of a 6-minute walking test. The clinical interview, physical examination findings, and transthoracic echocardiographic results served to rule out any of these previous conditions. Finally, once the patient was eligible to enter the study, block randomization was used, keeping in mind the permanent pacing indication (atrioventricular block or sick sinus syndrome). All patients provided informed consent, and the study was performed according to the Institutional Guidelines of the La Fe University Hospital.
The primary objective of the study was to compare the echocardiographic features (LV systolic function, LV volumes, and dyssynchrony parameters) in the 3 subgroups. The secondary objectives included the comparison of the clinical and biologic parameters (New York Heart Association Functional class, quality-of-life scores, as assessed by the European Quality of Life Scale [EuroQol] EQ-5D instrument, distance in the 6-minute walking test, and N-terminal pro-hormone brain natriuretic peptide [NT-proBNP] levels). Finally, a combined clinical end point, including new-onset atrial fibrillation or heart failure and heart failure hospitalizations, was also assessed at 12 months of follow-up. The diagnosis of new-onset heart failure was established using the modified Framingham criteria.
All procedures were performed by the same 2 implanters (JO and JEC) using direct subclavian puncture. Under fluoroscopic guidance, an active fixation (screw-in), bipolar and steroid-eluting pacing lead was introduced with a stylet and directed to the RVA or RVS, depending on randomization. In the RVSP group, an S -shaped preformed stylet similar to that described by Vlay was used to advance the lead toward the pulmonary valve. At that point, the lead was gently withdrawn to reach the right ventricular mid-septal zone. Two orthogonal fluoroscopic views (right anterior oblique and left anterior oblique) and electrocardiographic features were used to confirm the final mid-septal position, as previously described ( Figure 1 ). Ventricular leads from 4 different manufacturers (Selox SR, Biotronik, Berlin, Germany; Flextend 2, Guidant, Boston Scientific, Natick, Massachusetts; CapSureFix 5076, Medtronic, Minneapolis, Minnesota; and Tendril SDX, St. Jude Medical, Secaucus, New Jersey) were used, depending on the implanter’s preference, and were equally distributed between the 2 randomization arms.
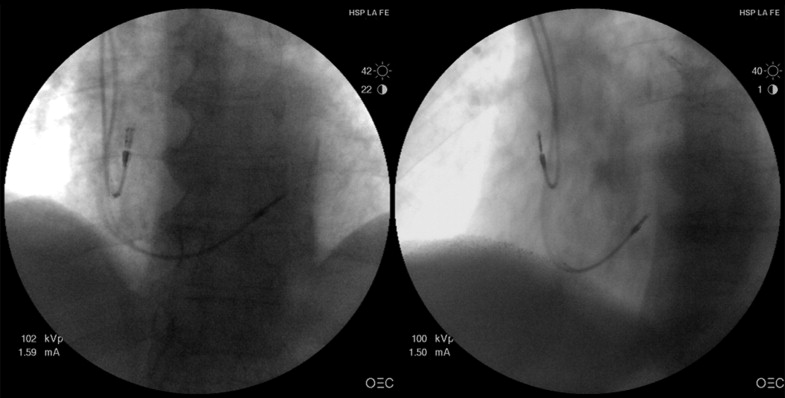
The patients were initially evaluated within the first week after implantation (baseline evaluation) and after 6 and 12 months of follow-up. All scheduled visits included a complete clinical interview, physical examination, 12-lead electrocardiogram, quality-of-life evaluation with the EuroQuol EQ-5D instrument, transthoracic echocardiography, 6-minute walking test, pacemaker interrogation, and measurement of NT-proBNP.
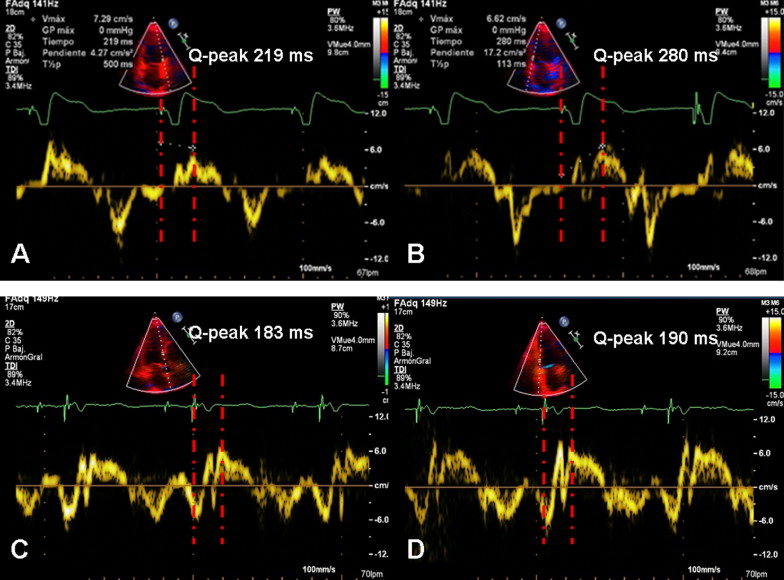
Images were obtained by the same single investigator using an iE33 model (Philips Medical Systems, Eindhoven, The Netherlands) with a 3.5-MHz transducer in the parasternal (long- and short-axis views) and apical (2-, 3-, and 4-chamber) views. The LV end-diastolic and end-systolic volumes and LV ejection fraction were calculated from the apical 2- and 4-chamber images using the biplane Simpson rule. Interventricular dyssynchrony was calculated as the difference between the left and right pre-ejection intervals measured with pulse Doppler echocardiography. The maximum difference of the time to the peak systolic velocity for the 6 segments at the basal level measured from the beginning of the QRS using pulse tissue Doppler imaging was considered the intraventricular dyssynchrony. Thus, the anterior-to-inferior wall delay, septal-to-posterior wall delay, and septal-to-lateral wall delay were measured in the apical 2-, 3-, and 4-chamber planes, respectively. All tissue Doppler imaging echocardiographic measurements were taken as averages of ≥3 representative cycles. Additionally, we considered the maximal delay to peak systolic velocity with tissue Doppler imaging between any of the 6 LV basal segments as a measurement of global asynchrony. All the echocardiographic evaluations in the control group were performed during intrinsic basal rhythm (not paced), and in the study subgroups (RVAP and RVSP) were performed during spontaneous paced rhythm. When native atrioventricular conduction was present at the echocardiographic evaluation, shortening of the atrioventricular interval was used to acutely ensure ventricular pacing. A stable heart rate was required to obtain reproducible measurements of LV dyssynchrony.
The continuous data are expressed as the mean ± SD or range, as appropriate. The categorical variables were compared using the chi-square test. One-way analysis of variance was used to compare the repeated measures of continuous variables between groups, followed by a post hoc Bonferroni’s test, as appropriate. p Values of ≤0.05 were considered statistically significant. All results were analyzed on an intention-to-treat basis.
Results
A total of 93 patients were enrolled from January 2006 to August 2007, with 46 patients randomized to RVAP and 47 to RVSP. Seven patients were lost to follow-up, because they refused to continue in the study after inclusion (3 in the RVAP group and 4 in the RVSP group). One patient in the RVSP group was excluded after developing an incapacitating hemorrhagic stroke in the third month of follow-up and another patient from the RVAP group was excluded because a malignant tumor diagnosis hampered the scheduled visits. Three patients died during the 12 months of follow-up, 2 in the RVSP and 1 in the RVAP group, none from cardiovascular causes. Thus, a total of 81 patients were analyzed, 40 randomized to RVAP and 41 to RVSP (53 men, mean age 72 ± 10 years, mean LVEF 63 ± 8%). Once the mean %VP was assessed at the baseline evaluation, a control group (n = 21) consisting of patients with a mean %VP of ≤10% was established. The patients with a %VP >10% were divided in 2 subgroups, depending on the location of the ventricular pacing lead: RVAP (n = 28) and RVSP (n = 32). Only 4 patients (14.3%) in the RVAP group and 3 patients (9.4%) in the RVSP group had preserved and stable native conduction. Programming changes during the echocardiographic evaluation to ensure ventricular pacing were necessary in 3 RVAP patients (10.7%) and 2 RVSP patients (6.2%). The baseline characteristics of the population are listed in Table 1 .
| Variable | Controls (n = 21) | RVAP (n = 28) | RVSP (n = 32) |
|---|---|---|---|
| Age (years) | 70 ± 11 | 72 ± 10 | 72 ± 9 |
| Men | 14 (67%) | 14 (50%) | 20 (63%) |
| New York Heart Association class | 1.2 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.6 |
| Atrial fibrillation | 4 (19%) | 6 (21%) | 7 (22%) |
| Pacing indication | |||
| Atrioventricular block | 2 (10%) | 26 (93%) | 31 (97%) |
| Sick sinus syndrome | 19 (90%) | 2 (7%) | 1 (3%) |
| Pacing mode | |||
| VVI | 3 (14%) | 3 (11%) | 4 (12%) |
| DDD | 18 (86%) | 25 (89%) | 28 (88%) |
| Hypertension ⁎ | 13 (62%) | 16 (57%) | 22 (71%) |
| Diabetes mellitus † | 0 (0%) | 12 (43%) | 7 (23%) |
| Hypercholesterolemia ‡ | 8 (38%) | 14 (50%) | 15 (48%) |
| Drug therapy at inclusion | |||
| Angiotensin-converting enzyme inhibitors | 7 (33%) | 12 (43%) | 15 (48%) |
| β Blockers | 5 (24%) | 5 (18%) | 2 (6.5%) |
| Diuretics | 4 (19%) | 9 (32%) | 12 (39%) |
| Calcium channel antagonists | 6 (29%) | 6 (21%) | 10 (32%) |
| Lipid-lowering agents | 7 (33%) | 12 (43%) | 14 (45%) |
⁎ Defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or if patient prescribed antihypertensive medication.
† Defined as serum fasting glucose of ≥7.0 mmol/L or taking medication.
‡ Defined as cholesterol of ≥6.4 mmol/L or treatment with lipid-lowering drugs.
The pacing and sensing parameters remained stable and comparable between the 2 locations during implantation and at the 6- and 12-month follow-up visits. Only 1 case of lead dislodgment requiring surgical reposition was registered in each randomization arm (p = NS). The paced QRS duration was significantly longer in patients in the RVAP group (mean paced QRS duration at 12 months 162.2 ± 15.1 ms for RVAP vs 151.3 ± 18.3 ms for the RVSP group, p <0.001).
The baseline clinical parameters and NT-proBNP values were comparable among the 3 groups ( Table 2 ). The RVAP group tended to have more interventricular dyssynchrony than the RVSP group, but the difference was not statistically significant (24.3 ± 18.1 ms for RVAP, 16.1 ± 14.3 ms for RVSP, and 20.5 ± 15.9 ms for controls, p = 0.055, between RVAP vs RVSP, using the Bonferroni test). The RVAP and RVSP groups had more intraventricular dyssynchrony at baseline than the control group, without differences in LV volume or LVEF ( Figure 2 and Table 3 ).
| Variable | Control Group (n = 21) | RVAP (n = 28) | RVSP (n = 32) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-mo Follow-Up | 12-mo Follow-Up | Baseline | 6-mo Follow-Up | 12-mo Follow-Up | Baseline | 6-mo Follow-Up | 12-mo Follow-Up | |
| NT-proBNP (pg/ml) | 438 ± 504 | 500 ± 717 | 269 ± 346 | 653 ± 718 | 483 ± 626 | 406 ± 438 | 700 ± 851 | 446 ± 430 | 442 ± 568 |
| 6-Minute walking test (m) | 413 ± 82 | 442 ± 64 | 459 ± 63 | 402 ± 91 | 428 ± 103 | 433 ± 95 | 389 ± 102 | 424 ± 96 | 427 ± 90 |
| New York Heart Association functional class | 1.16 ± 0.42 | 1.15 ± 0.28 | 1.18 ± 0.44 | 1.30 ± 0.43 | 1.31 ± 0.48 | 1.29 ± 0.44 | 1.45 ± 0.60 | 1.18 ± 0.42 | 1.25 ± 0.42 |
| EuroQol EQ-5D index | 0.94 ± 0.08 | 0.91 ± 0.11 | 0.90 ± 0.12 | 0.89 ± 0.28 | 0.86 ± 0.17 | 0.86 ± 0.22 | 0.91 ± 0.08 | 0.90 ± 0.10 | 0.90 ± 0.12 |
| EuroQol EQ-5D visual analogue scale | 0.79 ± 0.18 | 0.82 ± 0.11 | 0.86 ± 0.13 | 0.79 ± 0.14 | 0.80 ± 0.17 | 0.81 ± 0.17 | 0.77 ± 0.15 | 0.84 ± 0.13 | 0.83 ± 0.16 |
| Variable | Control Group (n = 21) | RVAP (n = 28) | RVSP (n = 32) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-mo Follow-Up | 12-mo Follow-Up | Baseline | 6-mo Follow-Up | 12-mo Follow-Up | Baseline | 6-mo Follow-Up | 12-mo Follow-Up | |
| Mean percentage of ventricular pacing | 4.9 ± 2.7 ⁎ † | 5.1 ± 3.5 ⁎ † | 5.2 ± 4.1 ⁎ † | 85.5 ± 19.2 ⁎ | 80.3 ± 28.8 ⁎ | 88.4 ± 17.1 ⁎ | 82.5 ± 24.5 † | 78.2 ± 26.6 † | 80.6 ± 25.9 † |
| RR interval (ms) § | 837 ± 92 | 824 ± 72 | 820 ± 72 | 849 ± 109 | 869 ± 91 | 868 ± 115 | 865 ± 107 | 862 ± 91 | 870 ± 76 |
| Left ventricular end-diastolic volume (ml) | 80.5 ± 24.1 | 86.2 ± 28.4 | 79.5 ± 22.4 | 88.6 ± 24.3 | 81.3 ± 23.8 | 79.5 ± 29.8 | 82.2 ± 22.1 | 83.9 ± 23.7 | 78.1 ± 21.4 |
| Left ventricular end-systolic volume (ml) | 29.2 ± 11.6 | 31.5 ± 11.3 | 26.8 ± 11.1 | 33.2 ± 12.9 | 31.9 ± 10.2 | 30.1 ± 14.5 | 29.1 ± 11.8 | 31.7 ± 9.8 | 25.8 ± 9.9 |
| Left ventricular ejection fraction (%) | 63.6 ± 7.2 | 62.6 ± 7.1 | 66.6 ± 7.2 | 62.9 ± 6.3 | 61.3 ± 8.6 | 62.9 ± 7.9 | 64.2 ± 8 | 63.2 ± 6.5 | 66.5 ± 7.2 |
| Interventricular delay (ms) | 20.5 ± 15.9 | 18.6 ± 11.5 | 17.4 ± 14.5 | 24.3 ± 18.1 | 28.9 ± 20.7 ⁎ ‡ | 31.5 ± 24.6 ⁎ | 16.1 ± 14.3 | 17.3 ± 13 ‡ | 24.1 ± 16.9 |
| Septal-to-posterior wall delay (peak) (ms) | 13.6 ± 13.7 | 21.6 ± 22.2 | 21.0 ± 22.9 | 33.2 ± 39.4 ⁎ | 33.4 ± 32.7 ‡ | 31.2 ± 33.6 | 39 ± 39.7 † | 20 ± 19.9 ‡ | 19.3 ± 18.8 |
| Septal-to-lateral wall delay (peak) (ms) | 16.0 ± 12.4 | 20.4 ± 22.7 | 17.2 ± 13.3 | 36.6 ± 37.5 ⁎ | 42.1 ± 38.8 ⁎ ‡ | 30.7 ± 27.1 ⁎ ‡ | 37.2 ± 36.9 † | 22.5 ± 19.9 ‡ | 14.9 ± 13.6 ‡ |
| Anterior-to-inferior wall delay (peak) (ms) | 27.4 ± 19.7 | 12.6 ± 10.7 | 12.5 ± 9.5 | 29.9 ± 26.1 | 37.2 ± 30.5 ⁎ | 26.9 ± 23.3 ⁎ ‡ | 25.6 ± 22.6 | 29.4 ± 24.9 | 13.9 ± 14.1 ‡ |
| Maximal delay to peak systolic velocity (ms) | 40.3 ± 17.0 | 37.6 ± 20.8 | 36.5 ± 17.8 | 68.3 ± 43.8 ⁎ | 62.4 ± 37.6 ⁎ ‡ | 57.8 ± 38.2 ⁎ ‡ | 59 ± 38.9 † | 45.3 ± 23.7 ‡ | 35.5 ± 20.6 ‡ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


