The aim of this study was to determine if earlier administration of oral β blocker therapy in patients with acute coronary syndromes (ACSs) is associated with an increased short-term survival rate and improved left ventricular (LV) function. We studied 11,581 patients enrolled in the International Survey of Acute Coronary Syndromes in Transitional Countries registry from January 2010 to June 2014. Of these patients, 6,117 were excluded as they received intravenous β blockers or remained free of any β blocker treatment during hospital stay, 23 as timing of oral β blocker administration was unknown, and 182 patients because they died before oral β blockers could be given. The final study population comprised 5,259 patients. The primary outcome was the incidence of in-hospital mortality. The secondary outcome was the incidence of severe LV dysfunction defined as an ejection fraction <40% at hospital discharge. Oral β blockers were administered soon (≤24 hours) after hospital admission in 1,377 patients and later (>24 hours) during hospital stay in the remaining 3,882 patients. Early β blocker therapy was significantly associated with reduced in-hospital mortality (odds ratio 0.41, 95% CI 0.21 to 0.80) and reduced incidence of severe LV dysfunction (odds ratio 0.57, 95% CI 0.42 to 0.78). Significant mortality benefits with early β blocker therapy disappeared when patients with Killip class III/IV were included as dummy variables. The results were confirmed by propensity score–matched analyses. In conclusion, in patients with ACSs, earlier administration of oral β blocker therapy should be a priority with a greater probability of improving LV function and in-hospital survival rate. Patients presenting with acute pulmonary edema or cardiogenic shock should be excluded from this early treatment regimen.
There is a general consensus that predischarge oral β blocker therapy leads to improved long-term clinical outcome in patients with acute coronary syndromes (ACSs) although within the framework of an in-hospital treatment strategy, there is a paucity of data on precisely defining when β blockers should be started. The most recent practice guidelines from the American College of Cardiology (ACC)/American Heart Association (AHA) recommend that oral β blocker therapy should be given in the first 24 hours if patients are at low risk for cardiogenic shock. Risk of cardiogenic shock, in turn, is based on findings from the COMMIT/CCS-2 (Chinese Clopidogrel and Metoprolol in Myocardial Infarction Trial/Second Chinese Cardiac Study) study. American recommendation is not reflected by the practice guidelines of the European Society of Cardiology where decisions on whether to give β blocker therapy within 24 hours from admission or several days later are left at physicians’ discretion. When solid evidence exists, guidelines tend to put forward largely overlapping recommendations. Further data are, therefore, needed on the relation between outcome and time to β blocker treatment in patients with ACS. The present study was undertaken to examine the effects of early versus late oral β blocker therapy in patients who had stabilized after an ACS.
Methods
The details of the International Survey of Acute Coronary Syndromes in Transitional Countries (ISACS-TC) registry protocol ( ClinicalTrials.gov : NCT01218776 ) have been previously published. Briefly, the ISACS-TC is both a retrospective, over a one year period, and prospective study which was designed to obtain data of patients with ACSs, and herewith, control and optimize internationally guideline recommended therapies in countries with economy in transition. Data collection activities began in October 2010 with the aim of collecting data on approximately 3,000 patients hospitalized with ACS on an annual basis. A total of 57 cluster sites in 11 countries in Central and Eastern Europe are currently collaborating in ISACS-TC ( Supplementary Material ). There were 29 tertiary health care services providing advanced medical investigation and treatment including percutaneous coronary intervention (PCI) and/or cardiac surgery, and 28 secondary health care services providing intensive care in critical coronary care units. The study was approved by the local research ethics committee from each hospital. Patients provided written consent for evaluation of their medical notes and monitoring of their health status.
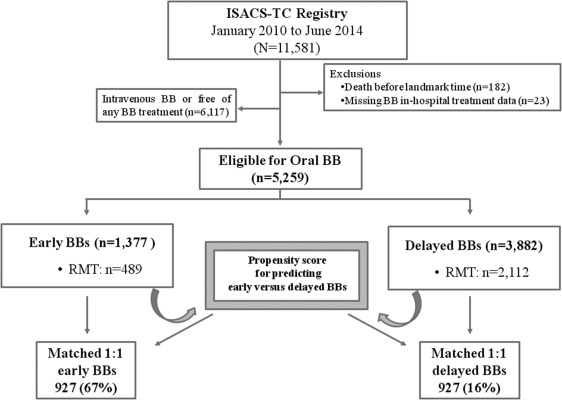
To avoid survival bias, as patients who were selected for the study would have to survive enough to received benefits from medications, a landmark time was used. We defined the landmark time as a 24-hour survival interval from β blocker administration. The analysis then evaluated patients’ outcome from the landmark time through to the end of the follow-up period (death or hospital discharge). Patients were also excluded from the analysis if they received intravenous β blockers or if they remained free of any β blocker treatment during hospital stay ( Figure 1 ).
The primary outcome was the incidence of in-hospital mortality. The secondary outcome was the incidence of severe left ventricular (LV) dysfunction defined as an ejection fraction by echocardiography <40% at hospital discharge. Moreover, to analyze the risk of shock as a potential confounder, the COMMIT-shock index score was calculated for each patient (0 to 2 points = low risk; 3 to 4 points = high risk). The shock index includes the following variables: age >70 years, symptom onset >12 hours, systolic blood pressure <120 mm Hg, and heart rate >110 beats/min.
Patients were stratified by time from hospital presentation to β blocker treatment whether early (≤24 hours) or delayed (>24 hours to discharge). Baseline characteristics, in-hospital therapies, and clinical outcomes were assessed. Patients were also stratified according to the index event: ST-segment elevation myocardial infarction (STEMI) versus non–ST-segment elevation ACS (NSTE-ACS) and in-hospital management strategies (overall population vs only routine medical therapy [RMT]). Standard initial routine medical therapies include the use of antiplatelet agents with aspirin and P2Y12 inhibitors, and anticoagulation with enoxaparin, bivalirudin, fondaparinux, or unfractionated heparin. In addition to standard initial antiplatelet/anticoagulant therapy, angiotensin-converting enzyme (ACE) inhibitors and β blockers could be started and continued indefinitely. Statistical testing was performed using a chi-square test for baseline categorical variables and a 2-sample t test for continuous variables. Estimates of the odds ratio (OR) and associated 95% CIs were obtained using the multivariate logistic regression analysis, adjusting the differences in baseline patient characteristics and medications given in the first 24 hours. Constant covariates included in the analyses were: (1) gender, (2) age and cardiovascular risk factors, (3) hypercholesterolemia, (4) diabetes mellitus, (5) hypertension, (6) current smoker, (7) family history of coronary artery disease (CAD), (8) clinical history of cardiovascular heart disease (previous angina pectoris, previous myocardial infarction, previous coronary artery bypass graft and PCI, previous heart failure, peripheral artery disease, and previous stroke), (9) chronic kidney disease, (10) time from symptom onset to admission <12 hours, and (11) STEMI as an index event. Covariates introduced as dummy variables were the use of fibrinolysis, aspirin, clopidogrel, heparins (unfractioned heparin) ACE inhibitors, and Killip class III/IV. For all analysis, statistical significance was defined as a value of p <0.05 and STATA 11 (StataCorp; College Station, Texas) was used.
Logistic regression analyses were used to obtain the estimated probabilities P and the logits (logit = ln(P/(1−P))), which were considered for the propensity score. The treatment variable (early β blocker treatment yes/no) was the outcome and the pretreatment covariates were the same 11 predictor variables entered in the previously mentioned multivariate models. We, then, assessed early β blocker versus delayed treatment effects by NCSS, version 9, routines for data matching (NCSS 9; NCSS LLC, Kaysville, Utah; www.ncss.com ). Patients were matched without replacement on a 1:1 basis using a nearest neighbor (Greedy) algorithm based on Mahalanobis distance.
Results
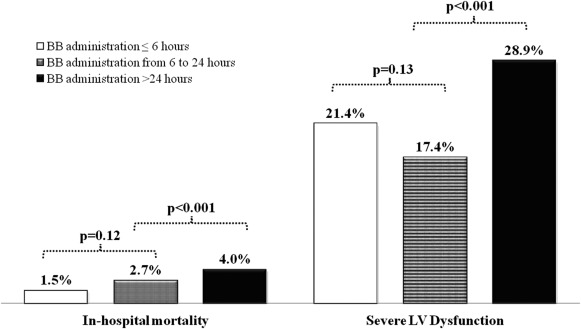
The baseline characteristics, in-hospital treatments, and outcomes of the overall study population (n = 5,259) and the RMT subgroup (n = 2,601) stratified according to time from hospital presentation to β blocker administration are listed in Table 1 . Average hospital stay was 7.8 ± 5.4 days in all patients and 8.2 ± 5.7 days in patients who underwent RMT. The corresponding values for median stay were, respectively, 7 and 7 days. Unadjusted in-hospital mortality was higher in the RMT group (4.8% vs 3.4%; OR 2.31, 95% CI 1.68 to 3.19, p <0.001). After adjustment RMT was still associated with higher mortality compared with that of the overall cohort (OR 1.65, 95% CI 1.08 to 2.51, p = 0.02). There were no significant differences in the in-hospital rates of death or severe LV dysfunction in patients who underwent β blocker therapy ≤6 hours versus those who had such therapy >6 to 24 hours after clinical presentation. In contrast, patients who underwent β blocker therapy >24 hours after clinical presentation had higher rates of death and severe LV dysfunction in comparison with those who underwent earlier therapy ( Figure 2 ). The relations between β blocker use ≤24 hours and subsequent end points are listed in Table 2 . The use of β blockers ≤24 hours was significantly associated with lower in-hospital mortality and lower incidence of severe LV dysfunction in the whole population. A qualitatively similar reduction in risk was seen in the RMT subgroup. After multivariate adjustment for demographic and clinical factors, early β blocker treatment remained a strong independent factor associated with better outcomes. Patients with STEMI treated with early β blockers had lower rates of adjusted in-hospital mortality and incidence of severe LV dysfunction. In the NSTE-ACS subgroup, the adjusted OR remained significantly associated with lower incidence of severe LV dysfunction, but not with decreased in-hospital mortality ( Figure 3 ). The adjusted OR associated with early β blocker therapy did not change when controlling for fibrinolytic, antiplatelet, and anticoagulant agents ( Table 2 ). Additional analysis revealed that the favorable outcomes associated with early β blocker treatment disappeared after adjustment for concurrent ACE inhibitors administration, suggesting an interaction between these 2 compounds. A regression model was, therefore, used to evaluate whether the treatment effects of these medications may have interacted. Beta blockers scored significantly for both lower incidence of both in-hospital mortality (OR 0.43, 95% CI 0.20 to 0.92, p = 0.03) and occurrence of severe LV dysfunction at discharge (OR 0.42, 95% CI 0.29 to 0.62, p <0.001). On the opposite, ACE inhibitors did not show significant effects either on death (OR 0.62, 95% CI 0.29 to 1.32, p = 0.21) or severe LV dysfunction (OR 1.44, 95% CI 0.98 to 2.14, p = 0.06). Significant benefits in mortality with early β blocker therapy disappeared when patients with Killip class >II were included in the analysis ( Table 2 ), indicating that patients with acute pulmonary edema or cardiogenic shock should be excluded from an early β blocker treatment regimen. We also investigated the relation between β adrenergic–blocker use and clinical correlates of LV function at hospital admission, using the COMMIT-shock index score. We calculated the COMMIT-shock index estimates only for patients with Killip class I/II, as for definition, patients with Killip class III/IV have shock or high risk for shock ( Table 3 ). In this lower risk population, approximately half of patients with STEMI (45%) and almost 2/3 of patients with NSTE-ACS (65%) had 2 or more risk factors for shock. Multivariate regression analysis indicated that all the individual factors entering the shock index were associated with increased in-hospital mortality rate. However, after adjustment for these factors, early β blocker use was still significantly associated with better outcomes ( Table 3 ). Delay to β blocker therapy >24 hours after clinical presentation was associated with significant increases in the rates of severe LV dysfunction in high shock risk score (2 or more factors) patients ( Figure 2 ). There were very few deaths (n = 23) in patients with Killip class I/II. Therefore, the increased risk of death with delayed β blocker therapy was not adequately powered to evaluate differences. Finally, the association between acute β blocker therapy and in-hospital clinical outcomes also was assessed using propensity score analysis The C-statistic for the propensity score logistic regression–based model was 0.70, thus indicating a good discriminatory power. Based on this propensity score, we matched 927 patients using a 1:1 model with 11 covariates for early versus delayed treatment ( Table 4 ). The logit propensity scores of early versus delayed β blocker administration were −0.252 ± 0.704 and −0.240 ± 0.692, respectively (p = not significant). The incidence of the in-hospital mortality was lower in patients with early β blocker treatment as compared with their delayed treatment counterpart: 1.2% (11 of 927) versus 2.7% (25 of 927, p = 0.018).
| Variable | Overall population | Routine medical therapy | p value ‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=5,259) | Early β Blockers administration (n=1,377) | Delayed β Blockers administration (n=3,882) | p value ∗ | All (n=2,601) | Early β Blockers administration (n=489) | Delayed β Blockers administration (n=2,112) | p value † | ||
| Women | 1,681 (31.9%) | 431 (31.3%) | 1,250 (32.2%) | 0.53 | 942 (36.2%) | 188 (38.5%) | 754 (35.7%) | 0.25 | <0.001 |
| Age (years) | 62.1 ± 11.9 | 61.8 ± 11.7 | 62.2 ± 12.1 | 0.33 | 64.0 ± 12.3 | 65.7 ± 11.7 | 63.6 ± 12.4 | <0.001 | <0.001 |
| Hypercholesterolemia | 2,010 (46.8%) | 595 (57.0%) | 1,415 (43.5%) | <0.001 | 885 (43.4%) | 162 (58.5%) | 723 (41.0%) | <0.001 | 0.01 |
| Diabetes mellitus | 1,256 (25.0%) | 313 (23.1%) | 943 (25.7%) | 0.06 | 680 (27.8%) | 141(29.1%) | 539 (27.5%) | 0.46 | 0.009 |
| Hypertension | 3,570 (69.5%) | 993 (75.0%) | 2,577 (67.6%) | <0.001 | 1,768 (69.8%) | 352 (77.9%) | 1,416 (68.0%) | <0.001 | 0.78 |
| Current smoker | 1,790 (34.6%) | 584 (43.4%) | 1,206 (31.5%) | <0.001 | 744 (29.2%) | 174 (36.6%) | 570 (27.5%) | <0.001 | <0.001 |
| Former smoker | 408 (7.9%) | 157 (11.7%) | 251 (6.6%) | <0.001 | 218 (8.6%) | 49 (10.3%) | 169 (8.2%) | 0.12 | 0.28 |
| Family history of CAD | 2,032 (41.8%) | 378 (29.8%) | 1,654 (46.0%) | <0.001 | 878 (36.4%) | 103 (23.5%) | 775 (39.2%) | <0.001 | <0.001 |
| Prior angina pectoris | 1,361 (25.9%) | 495 (35.9%) | 866 (22.3%) | <0.001 | 721 (27.7%) | 225 (46.0%) | 496 (23.5%) | <0.001 | 0.08 |
| Peripheral artery disease | 165 (3.1%) | 24 (1.7%) | 141 (3.6%) | 0.001 | 106 (4.1%) | 12 (2.5%) | 94 (4.5%) | 0.04 | 0.02 |
| Prior myocardial infarction | 921 (17.5%) | 185 (13.4%) | 736 (18.9%) | <0.001 | 544 (20.9%) | 96 (19.6%) | 448 (21.2%) | 0.43 | <0.001 |
| Prior coronary artery bypass graft | 156 (3.0%) | 27 (1.9%) | 129 (3.2%) | 0.01 | 104 (4.0%) | 17 (3.5%) | 87 (4.1%) | 0.51 | 0.009 |
| Prior percutaneous coronary intervention | 989 (18.8%) | 86 (6.3%) | 903 (23.3%) | <0.001 | 330 (12.7%) | 34 (6.9%) | 296 (14.0%) | <0.001 | <0.001 |
| Prior heart failure | 263 (5.0%) | 160 (11.6%) | 103 (2.7%) | <0.001 | 159 (6.1%) | 75 (15.3%) | 84 (4.0%) | <0.001 | 0.04 |
| Prior stroke | 268 (5.1%) | 53 (3.8%) | 215 (5.5%) | 0.01 | 163 (6.3%) | 30 (6.1%) | 133 (6.3%) | 0.89 | 0.03 |
| Chronic kidney disease | 278 (5.3%) | 75 (5.5%) | 203 (5.3%) | 0.80 | 180 (7.0%) | 42 (8.6%) | 138 (6.6%) | 0.11 | <0.001 |
| Killip class I and II | 4,840 (92.0%) | 1,324 (96.2%) | 3,516 (90.6%) | <0.001 | 2,294 (88.2%) | 453 (92.6%) | 1,841 (87.2%) | 0.001 | <0.001 |
| Time from symptom onset to admission ≤12 hours | 3,420 (70.3%) | 994 (74.1%) | 2,426 (68.8%) | <0.001 | 1,488 (61.9%) | 308 (66.2%) | 1,180 (60.9%) | 0.03 | <0.001 |
| Serum Creatinine (μmol/L) | 95.5 ± 73.5 | 92.2 ± 61.7 | 104.2 ± 97.4 | 0.002 | 104.6 ± 86.3 | 99.1 ± 54.7 | 112.9 ± 118.6 | 0.02 | 0.006 |
| Heart rate (beats/min) | 82.3 ± 25.5 | 82.9 ± 22.8 | 81.9 ± 27.5 | 0.28 | 83.9 ± 24.9 | 87.4 ± 30.9 | 82.4 ± 21.8 | <0.001 | 0.05 |
| Systolic blood pressure (mm Hg) | 140.5 ± 26.9 | 143.6 ± 25.3 | 137.9 ± 28.0 | <0.001 | 140.3 ± 27.7 | 144.1 ± 25.3 | 138.7 ± 28.4 | <0.001 | 0.71 |
| Index event | |||||||||
| STEMI | 3,742 (71.2%) | 846 (61.4%) | 2,896 (74.6%) | <0.001 | 1,595 (61.3%) | 202(41.3%) | 1,393 (65.9%) | <0.001 | <0.001 |
| NSTE-ACS | 1,517 (28.8%) | 531 (38.6%) | 986 (25.4%) | <0.001 | 1,006 (38.7%) | 287 (58.7%) | 719 (34.0%) | <0.001 | <0.001 |
| In-hospital acute medications (within 24 hours) | |||||||||
| Fibrinolytic therapy | 767 (14.6%) | 122 (8.9) % | 645 (16.7%) | <0.001 | 557 (21.5%) | 85 (17.4%) | 472 (22.4%) | 0.01 | <0.001 |
| Aspirin | 5,126 (97.8%) | 1,363 (99.1%) | 3,763 (97.3%) | <0.001 | 2,498 (96.3%) | 479 (98.2%) | 2,019 (95.8%) | 0.01 | <0.001 |
| Clopidogrel | 4642 (88.9%) | 1,321 (96.2%) | 3,321 (86.3%) | <0.001 | 2,060 (79.7%) | 450 (92.6%) | 1,610 (76.7%) | <0.001 | <0.001 |
| Unfractioned heparins | 2379 (46.1%) | 729 (53.1%) | 1,650 (43.5%) | <0.001 | 935 (36.8%) | 120 (24.6%) | 815 (39.7%) | <0.001 | <0.001 |
| Low molecular weight heparins | 1857 (41.9%) | 880 (64.2%) | 977 (31.9%) | <0.001 | 1,131 (49.9%) | 403 (82.8%) | 728 (40.9%) | <0.001 | <0.001 |
| Fondaparinux | 70 (1.6%) | 19 (1.4%) | 51 (1.7%) | 0.48 | 46 (2.0%) | 3 (0.6%) | 43 (2.4%) | 0.01 | 0.23 |
| Glycoprotein IIb/IIIa inhibitors | 225 (7.4%) | 87 (6.4%) | 138 (8.2%) | 0.05 | 20 (1.2%) | 1 (0.2%) | 19 (1.6%) | 0.01 | <0.001 |
| β blockers | 1377 (26.2%) | 1,377 (100%) | – | – | 489 (18.8%) | 489 (100.0%) | – | – | <0.001 |
| ACE inhibitor | 1244 (27.7%) | 1,156 (95.2%) | 88 (2.7%) | <0.001 | 432 (19.6%) | 404 (96.4%) | 28 (1.6%) | <0.001 | <0.001 |
| In-hospital procedures | |||||||||
| Coronary angiography | 2866 (55.7%) | 994 (75.7%) | 1,872 (48.9%) | <0.001 | 266 (10.5%) | 115 (26.6%) | 151 (7.2%) | <0.001 | <0.001 |
| Percutaneous coronary intervention | 2585 (49.3%) | 878 (64.2%) | 1,707 (44.7%) | <0.001 | – | – | – | – | – |
| Coronary artery bypass graft | 21 (0.4%) | 7 (0.5%) | 14 (0.4%) | 0.45 | – | – | – | – | – |
| Outcomes | |||||||||
| In-hospital mortality | 179 (3.4%) | 25 (1.8%) | 154 (4.0%) | <0.001 | 124 (4.8%) | 17 (3.5%) | 107 (5.1%) | 0.13 | 0.002 |
| Severe LV dysfunction | 546 (24.4%) | 237 (20.3%) | 309 (28.9%) | <0.001 | 323 (27.8%) | 104 (24.2%) | 219 (29.8%) | 0.04 | 0.03 |
∗ p Value derived from comparison between early versus delayed β blocker administration in the overall population.
† p Value derived from comparison between early versus delayed β blocker administration in the routine medical therapy subgroup.
‡ p Value derived from comparison between β blocker administration in the overall population versus routine medical therapy subgroup.
| Multivariate adjusted models: | Overall population | Routine medical therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | p value | N | OR | 95% CI | p value | |
| Model 1: Demographic and clinical factors ∗ | ||||||||
| In-hospital mortality | 3,557 | 0.41 | 0.21 – 0.80 | 0.01 | 1,681 | 0.75 | 0.29 – 1.92 | 0.56 |
| Severe LV dysfunction | 1,421 | 0.57 | 0.42 – 0.78 | <0.001 | 659 | 0.37 | 0.20 – 0.67 | 0.001 |
| Model 2: Model 1 including Fibrinolysis | ||||||||
| In-hospital mortality | 3,544 | 0.39 | 0.20 – 0.77 | 0.007 | 1,676 | 0.74 | 0.29 – 1.88 | 0.52 |
| Severe LV dysfunction | 1,418 | 0.55 | 0.40 – 0.75 | <0.001 | 659 | 0.37 | 0.20 – 0.66 | 0.001 |
| Model 3: Model 1 including Fibrinolysis and Aspirin | ||||||||
| In-hospital mortality | 3,535 | 0.40 | 0.20 – 0.78 | 0.008 | 1,673 | 0.76 | 0.30 – 1.94 | 0.57 |
| Severe LV dysfunction | 1,415 | 0.55 | 0.40 – 0.75 | <0.001 | 657 | 0.35 | 0.19 – 0.64 | 0.001 |
| Model 4: Model 1 including Fibrinolysis, Aspirin and/or Clopidogrel | ||||||||
| In-hospital mortality | 3,544 | 0.41 | 0.21 – 0.80 | 0.01 | 1,676 | 0.77 | 0.30 – 1.94 | 0.58 |
| Severe LV dysfunction | 1,418 | 0.56 | 0.41 – 0.76 | <0.001 | 659 | 0.37 | 0.21 – 0.67 | 0.001 |
| Model 5: Model 1 including Fibrinolysis, Aspirin and/or Clopidogrel, and Unfractioned heparin | ||||||||
| In- hospital mortality | 3,496 | 0.37 | 0.19 – 0.73 | 0.004 | 1,638 | 0.77 | 0.30-1.98 | 0.60 |
| Severe LV dysfunction | 1,397 | 0.54 | 0.39 – 0.75 | <0.001 | 642 | 0.34 | 0.18 -0.63 | 0.001 |
| Model 6: Model 1 including Fibrinolysis, Aspirin and/or Clopidogrel, Unfractioned heparin and ACE-Inhibitors | ||||||||
| In-hospital mortality | 1,308 | 0.57 | 0.19 – 1.71 | 0.32 | 447 | 1.97 | 0.47 – 8.22 | 0.35 |
| Severe LV dysfunction | 848 | 0.59 | 0.34 – 1.02 | 0.06 | 267 | 0.28 | 0.11 – 0.74 | 0.01 |
| Model 7: Model 1 including Killip class III/ IV | ||||||||
| In-hospital mortality | 3,557 | 0.53 | 0.27 – 1.04 | 0.06 | 1,681 | 0.93 | 0.36 – 2.38 | 0.88 |
| Severe LV dysfunction | 1,421 | 0.68 | 0.50 – 0.92 | 0.01 | 659 | 0.46 | 0.24 – 0.83 | 0.01 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


